Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver
- PMID: 29280273
- PMCID: PMC5900872
- DOI: 10.1111/joim.12719
Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver
Abstract
Background and aims: Nonalcoholic fatty liver disease is epidemiologically associated with hepatic and metabolic disorders. The aim of this study was to examine whether hepatic fat accumulation has a causal role in determining liver damage and insulin resistance.
Methods: We performed a Mendelian randomization analysis using risk alleles in PNPLA3, TM6SF2, GCKR and MBOAT7, and a polygenic risk score for hepatic fat, as instruments. We evaluated complementary cohorts of at-risk individuals and individuals from the general population: 1515 from the liver biopsy cohort (LBC), 3329 from the Swedish Obese Subjects Study (SOS) and 4570 from the population-based Dallas Heart Study (DHS).
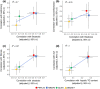
Results: Hepatic fat was epidemiologically associated with liver damage, insulin resistance, dyslipidemia and hypertension. The impact of genetic variants on liver damage was proportional to their effect on hepatic fat accumulation. Genetically determined hepatic fat was associated with aminotransferases, and with inflammation, ballooning and fibrosis in the LBC. Furthermore, in the LBC, the causal association between hepatic fat and fibrosis was independent of disease activity, suggesting that a causal effect of long-term liver fat accumulation on liver disease is independent of inflammation. Genetically determined hepatic steatosis was associated with insulin resistance in the LBC and SOS. However, this association was dependent on liver damage severity. Genetically determined hepatic steatosis was associated with liver fibrosis/cirrhosis and with a small increase in risk of type 2 diabetes in publicly available databases.
Conclusion: These data suggest that long-term hepatic fat accumulation plays a causal role in the development of chronic liver disease.
Keywords: fibrosis; genetics; insulin resistance; mendelian randomization; nonalcoholic fatty liver disease; type 2 diabetes.
© 2017 The Authors Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Figures


References
-
- Browning JD, Szczepaniak LS, Dobbins R, et al Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40: 1387–95. - PubMed
-
- Non‐alcoholic Fatty Liver Disease Study G , Lonardo A, Bellentani S, et al Epidemiological modifiers of non‐alcoholic fatty liver disease: Focus on high‐risk groups. Dig Liver Dis 2015; 47: 997–1006. - PubMed
-
- Valenti L, Bugianesi E, Pajvani U, Targher G. Nonalcoholic fatty liver disease: cause or consequence of type 2 diabetes? Liver Int 2016; 36: 1563–79. - PubMed
-
- Dyson J, Jaques B, Chattopadyhay D, et al Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol 2014; 60: 110–7. - PubMed
-
- Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011; 141: 1249–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

