The potential of the CMB305 vaccine regimen to target NY-ESO-1 and improve outcomes for synovial sarcoma and myxoid/round cell liposarcoma patients
- PMID: 29280411
- PMCID: PMC6521962
- DOI: 10.1080/14760584.2018.1419068
The potential of the CMB305 vaccine regimen to target NY-ESO-1 and improve outcomes for synovial sarcoma and myxoid/round cell liposarcoma patients
Abstract
Introduction: Synovial Sarcoma (SS) and Myxoid Round Cell Liposarcoma (MRCL) are devastating sarcoma subtypes with few treatment options and poor outcomes in the advanced setting. However, both these diseases may be ideal for novel immunotherapies targeting the cancer-testis antigen, NY-ESO-1.
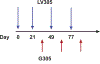
Areas covered: In this review, we discuss the novel NY-ESO-1 targeted vaccine regimen, CMB305. This regimen uses a unique integration-deficient, dendritic-cell targeting lentiviral vector from the ZVex® platform, LV305, in order to prime NY-ESO-1 specific T cells. LV305 has single agent activity, and, in one case, caused a durable partial response in a refractory SS patient. CMB305 also includes a boost from a NY-ESO-1 protein vaccine given along with a potent toll-like-4 receptor agonist, glycopyranosyl lipid A. CMB305 induces NY-ESO-1 specific T cell responses in both SS and MRC patients and these patients had excellent overall survival (OS) outcomes in the initial phase I study.
Expert commentary: CMB305 is a therapeutic vaccine regimen targeting NY-ESO-1 based on the lentiviral vaccine vector, LV305. Phase I studies have proven this vaccine is active immunologically. Data suggesting this vaccine may improve OS for SS and MRCL patients is exciting but early, and on-going work is testing the impact of CMB305 on patient outcomes.
Keywords: CMB305; LV305; Myxoid; NY-ESO-1; Sarcoma; Synovial; dendritic cells; immunotherapy; therapeutic vaccine.
Conflict of interest statement
Declaration of interest
Figures

References
-
- Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–1545. - PubMed
-
- Judson I, Verweij J, Gelderblom H, et al. European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014;15(4):415–423. - PubMed
-
- van der Graaf WT, Blay J-Y, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–1886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
