3,4-diaminopyridine base effectively treats the weakness of Lambert-Eaton myasthenia
- PMID: 29280483
- PMCID: PMC5900968
- DOI: 10.1002/mus.26052
3,4-diaminopyridine base effectively treats the weakness of Lambert-Eaton myasthenia
Abstract
Introduction: 3,4-diaminopyridine has been used to treat Lambert-Eaton myasthenia (LEM) for 30 years despite the lack of conclusive evidence of efficacy.
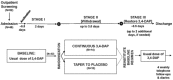
Methods: We conducted a randomized double-blind placebo-controlled withdrawal study in patients with LEM who had been on stable regimens of 3,4-diaminopyridine base (3,4-DAP) for ≥ 3 months. The primary efficacy endpoint was >30% deterioration in triple timed up-and-go (3TUG) times during tapered drug withdrawal. The secondary endpoint was self-assessment of LEM-related weakness (W-SAS).
Results: Thirty-two participants were randomized to continuous 3,4-DAP or placebo groups. None of the 14 participants who received continuous 3,4-DAP had > 30% deterioration in 3TUG time versus 72% of the 18 who tapered to placebo (P < 0.0001). W-SAS similarly demonstrated an advantage for continuous treatment over placebo (P < 0.0001). Requirement for rescue and adverse events were more common in the placebo group.
Discussion: This trial provides significant evidence of efficacy of 3,4-DAP in the maintenance of strength in LEM. Muscle Nerve 57: 561-568, 2018.
Keywords: 3,4-diaminopyridine; ELS; Eaton-Lambert syndrome; LEMS; LES; Lambert-Eaton myasthenia; Lambert-Eaton myasthenic syndrome; Lambert-Eaton syndrome; amifampridine; clinical trial; efficacy; timed up-and-go.
© 2017 The Authors Muscle & Nerve Published by Wiley Periodicals, Inc.
Figures

Comment in
-
3,4-diaminopyridine in Lambert-Eaton myasthenic syndrome: Concerns regarding presentation of previous studies.Muscle Nerve. 2018 May;57(5):E130. doi: 10.1002/mus.26091. Epub 2018 Feb 12. Muscle Nerve. 2018. PMID: 29406572 No abstract available.
-
Reply.Muscle Nerve. 2018 May;57(5):E130-E131. doi: 10.1002/mus.26089. Epub 2018 Mar 1. Muscle Nerve. 2018. PMID: 29406616 No abstract available.
References
-
- Juel VC, Sanders DB. Lambert‐Eaton myasthenic syndrome In: Engel AG, editor. Myasthenia gravis and myasthenic disorders, 2nd ed. Oxford, United Kingdom: Oxford University Press; 2015. p 156–172.
-
- Deenen JCW, Horlings CGC, Verschuuren JJGM, Verbeek ALM. The edipemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscular Dis 2015;2:73–85. - PubMed
-
- Abenroth DC, Smith AG, Greenlee JE, Austin SD, Clardy SL. Lambert–Eaton myasthenic syndrome: epidemiology and therapeutic response in the national veterans affairs population. Muscle Nerve 2017;56(3):421–426. - PubMed
-
- Lang B, Waterman S, Pinto A, Jones D, Moss F, Boot J, et al The role of autoantibodies in Lambert‐Eaton myasthenic syndrome. Ann N Y Acad Sci 1998;841:596–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

