Β-N-Methylamino-L-Alanine (BMAA) Toxicity Is Gender and Exposure-Age Dependent in Rats
- PMID: 29280981
- PMCID: PMC5793103
- DOI: 10.3390/toxins10010016
Β-N-Methylamino-L-Alanine (BMAA) Toxicity Is Gender and Exposure-Age Dependent in Rats
Abstract
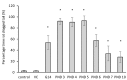
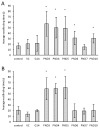
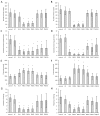
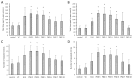
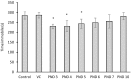
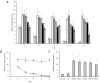
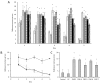
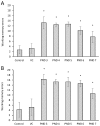
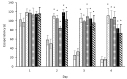
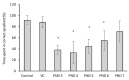
Cyanobacterial β-N-methylamino-L-alanine (BMAA) has been suggested as a causative or contributory factor in the development of several neurodegenerative diseases. However, no BMAA animal model has adequately shown clinical or behavioral symptoms that correspond to those seen in either Alzheimer's Disease (AD), Amyotrophic Lateral Sclerosis (ALS) or Parkinson's Disease (PD). We present here the first data that show that when neonatal rats were exposed to BMAA on postnatal days 3, 4 and 5, but not on gestational day 14 or postnatally on days 7 or 10, several AD and/or PD-related behavioral, locomotor and cognitive deficits developed. Male rats exhibited severe unilateral hindlimb splay while whole body tremors could be observed in exposed female rats. BMAA-exposed rats failed to identify and discriminate a learned odor, an early non-motor symptom of PD, and exhibited decreased locomotor activity, decreased exploration and increased anxiety in the open field test. Alterations were also observed in the rats' natural passive defense mechanism, and potential memory deficits and changes to the rat's natural height avoidance behavior could be observed as early as PND 30. Spatial learning, short-term working, reference and long-term memory were also impaired in 90-day-old rats that had been exposed to a single dose of BMAA on PND 3-7. These data suggest that BMAA is a developmental neurotoxin, with specific target areas in the brain and spinal cord.
Keywords: BMAA; behavior; cognition; motor function; neurodegeneration; rat; rats; β-N-methylamino-L-alanine.
Conflict of interest statement
The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
A Single Neonatal Exposure to BMAA in a Rat Model Produces Neuropathology Consistent with Neurodegenerative Diseases.Toxins (Basel). 2017 Dec 29;10(1):22. doi: 10.3390/toxins10010022. Toxins (Basel). 2017. PMID: 29286334 Free PMC article.
-
Neurochemical and neurobehavioral effects of neonatal administration of beta-N-methylamino-L-alanine and 3,3'-iminodipropionitrile.Neurotoxicol Teratol. 1998 Mar-Apr;20(2):181-92. doi: 10.1016/s0892-0362(97)00078-0. Neurotoxicol Teratol. 1998. PMID: 9536463
-
Early hippocampal cell death, and late learning and memory deficits in rats exposed to the environmental toxin BMAA (β-N-methylamino-L-alanine) during the neonatal period.Behav Brain Res. 2011 Jun 1;219(2):310-20. doi: 10.1016/j.bbr.2011.01.056. Epub 2011 Feb 12. Behav Brain Res. 2011. PMID: 21315110
-
A critical review of the postulated role of the non-essential amino acid, β-N-methylamino-L-alanine, in neurodegenerative disease in humans.J Toxicol Environ Health B Crit Rev. 2017;20(4):1-47. doi: 10.1080/10937404.2017.1297592. Epub 2017 Jun 9. J Toxicol Environ Health B Crit Rev. 2017. PMID: 28598725 Free PMC article. Review.
-
How does the neurotoxin β-N-methylamino-L-alanine exist in biological matrices and cause toxicity?Sci Total Environ. 2024 Apr 20;922:171255. doi: 10.1016/j.scitotenv.2024.171255. Epub 2024 Feb 26. Sci Total Environ. 2024. PMID: 38417517 Review.
Cited by
-
Dose-Dependent Adult Neurodegeneration in a Rat Model After Neonatal Exposure to β-N-Methylamino-L-Alanine.Neurotox Res. 2019 Apr;35(3):711-723. doi: 10.1007/s12640-019-9996-5. Epub 2019 Jan 21. Neurotox Res. 2019. PMID: 30666559
-
Experimental Models of Cognitive Impairment for Use in Parkinson's Disease Research: The Distance Between Reality and Ideal.Front Aging Neurosci. 2021 Nov 29;13:745438. doi: 10.3389/fnagi.2021.745438. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34912207 Free PMC article. Review.
-
Environmental Neurotoxin β-N-Methylamino-L-alanine (BMAA) as a Widely Occurring Putative Pathogenic Factor in Neurodegenerative Diseases.Microorganisms. 2022 Dec 6;10(12):2418. doi: 10.3390/microorganisms10122418. Microorganisms. 2022. PMID: 36557671 Free PMC article. Review.
-
A Single Neonatal Exposure to BMAA in a Rat Model Produces Neuropathology Consistent with Neurodegenerative Diseases.Toxins (Basel). 2017 Dec 29;10(1):22. doi: 10.3390/toxins10010022. Toxins (Basel). 2017. PMID: 29286334 Free PMC article.
-
The cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) targets the olfactory bulb region.Arch Toxicol. 2020 Aug;94(8):2799-2808. doi: 10.1007/s00204-020-02775-6. Epub 2020 May 20. Arch Toxicol. 2020. PMID: 32435914 Free PMC article.
References
-
- Dietrich D.R., Fischer A., Michel C., Hoeger S.J. Toxin mixture in cyanobacterial bloom—A critical comparison of reality with current procedures employed in human health risk assessment. Cyanobact. Harmful Algal Blooms. 2008;619:885–912. - PubMed
-
- Jonasson S., Eriksson J., Berntzon L., Spáčil Z., Ilag L.L., Ronnevi L.-O., Rasmussen U., Bergman B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. USA. 2010;107:9252–9257. doi: 10.1073/pnas.0914417107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

