Impaired PGE2-stimulated Cl- and HCO3- secretion contributes to cystic fibrosis airway disease
- PMID: 29281691
- PMCID: PMC5744969
- DOI: 10.1371/journal.pone.0189894
Impaired PGE2-stimulated Cl- and HCO3- secretion contributes to cystic fibrosis airway disease
Abstract
Background: Airway mucociliary clearance (MCC) is an important defense mechanism against pulmonary infections and is compromised in cystic fibrosis (CF). Cl- and HCO3- epithelial transport are integral to MCC. During pulmonary infections prostaglandin E2 (PGE2) production is abundant.
Aim: To determine the effect of PGE2 on airway Cl- and HCO3- secretion and MCC in normal and CF airways.
Methods: We examined PGE2 stimulated MCC, Cl- and HCO3- secretion using ferret trachea, human bronchial epithelial cell cultures (CFBE41o- with wildtype CFTR (CFBE41 WT) or homozygous F508del CFTR (CFBE41 CF) and human normal bronchial submucosal gland cell line (Calu-3) in Ussing chambers with or without pH-stat.
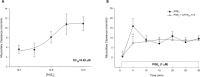
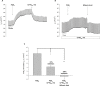
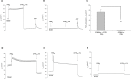
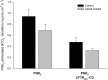
Results: PGE2 stimulated MCC in a dose-dependent manner and was partially impaired by CFTRinh-172. PGE2-stimulated Cl- current in ferret trachea was partially inhibited by CFTRinh-172, with niflumic acid eliminating the residual current. CFBE41 WT cell monolayers produced a robust Cl- and HCO3- secretory response to PGE2, both of which were completely inhibited by CFTRinh-172. CFBE41 CF cells exhibited no response to PGE2. In Calu-3 cells, PGE2 stimulated Cl- and HCO3- secretion. Cl- secretion was partially inhibited by CFTRinh-172, with additional inhibition by niflumic acid. HCO3- secretion was completely inhibited by CFTRinh-172.
Conclusions: PGE2 stimulates bronchotracheal MCC and this response is decreased in CF. In CF airway, PGE2-stimulated Cl- and HCO3- conductance is impaired and may contribute to decreased MCC. There remains a CFTR-independent Cl- current in submucosal glands, which if exploited, could represent a means of improving airway Cl- secretion and MCC in CF.
Conflict of interest statement
Figures



References
-
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571–7. doi: 10.1172/JCI15217 ; PubMed Central PMCID: PMC150901. - DOI - PMC - PubMed
-
- Jeong JH, Joo NS, Hwang PH, Wine JJ. Mucociliary clearance and submucosal gland secretion in the ex vivo ferret trachea. American journal of physiology Lung cellular and molecular physiology. 2014;307(1):L83–93. doi: 10.1152/ajplung.00009.2014 . - DOI - PubMed
-
- Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126(3):879–91. doi: 10.1172/JCI83922 ; PubMed Central PMCID: PMCPMC4767348. - DOI - PMC - PubMed
-
- Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209(7):1263–72. Epub 2012/06/20. doi: 10.1084/jem.20120562 ; PubMed Central PMCID: PMC3405509. - DOI - PMC - PubMed
-
- Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax. 2016;71(3):284–7. doi: 10.1136/thoraxjnl-2015-207588 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

