Serine-Dependent Sphingolipid Synthesis Is a Metabolic Liability of Aneuploid Cells
- PMID: 29281829
- PMCID: PMC5747309
- DOI: 10.1016/j.celrep.2017.11.103
Serine-Dependent Sphingolipid Synthesis Is a Metabolic Liability of Aneuploid Cells
Abstract
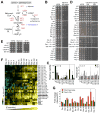
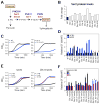
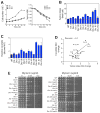
Aneuploidy disrupts cellular homeostasis. However, the molecular mechanisms underlying the physiological responses and adaptation to aneuploidy are not well understood. Deciphering these mechanisms is important because aneuploidy is associated with diseases, including intellectual disability and cancer. Although tumors and mammalian aneuploid cells, including several cancer cell lines, show altered levels of sphingolipids, the role of sphingolipids in aneuploidy remains unknown. Here, we show that ceramides and long-chain bases, sphingolipid molecules that slow proliferation and promote survival, are increased by aneuploidy. Sphingolipid levels are tightly linked to serine synthesis, and inhibiting either serine or sphingolipid synthesis can specifically impair the fitness of aneuploid cells. Remarkably, the fitness of aneuploid cells improves or deteriorates upon genetically decreasing or increasing ceramides, respectively. Combined targeting of serine and sphingolipid synthesis could be exploited to specifically target cancer cells, the vast majority of which are aneuploid.
Keywords: aneuploidy; ceramide; chromosomes; genomic istability; long-chain bases; metabolism; myriocin; serine; sphingolipids; sphingosine.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
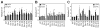

Comment in
-
Sphingolipid Turnover Turns Over the Fate of Aneuploid Cells.Trends Genet. 2018 Apr;34(4):255-256. doi: 10.1016/j.tig.2018.02.004. Epub 2018 Feb 21. Trends Genet. 2018. PMID: 29477508
References
-
- Alvarez-Vasquez F, Sims KJ, Cowart LA, Okamoto Y, Voit EO, Hannun YA. Simulation and validation of modelled sphingolipid metabolism in Saccharomyces cerevisiae. Nature. 2005;433:425–430. - PubMed
-
- Beeler TJ, Fu D, Rivera J, Monaghan E, Gable K, Dunn TM. SUR1 (CSG1/BCL21), a gene necessary for growth of Saccharomyces cerevisiae in the presence of high Ca2+ concentrations at 37 degrees C, is required for mannosylation of inositolphosphorylceramide. Mol Gen Genet. 1997;255:570–579. - PubMed
-
- Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol Biol. 2009;579:443–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

