Normothermic machine perfusion of donor livers without the need for human blood products
- PMID: 29281862
- PMCID: PMC5900573
- DOI: 10.1002/lt.25005
Normothermic machine perfusion of donor livers without the need for human blood products
Erratum in
-
Erratum.Liver Transpl. 2018 Aug;24(8):1151. doi: 10.1002/lt.25199. Liver Transpl. 2018. PMID: 30142247 Free PMC article. No abstract available.
Abstract
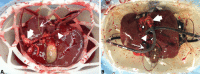
Normothermic machine perfusion (NMP) enables viability assessment of donor livers prior to transplantation. NMP is frequently performed by using human blood products including red blood cells (RBCs) and fresh frozen plasma (FFP). Our aim was to examine the efficacy of a novel machine perfusion solution based on polymerized bovine hemoglobin-based oxygen carrier (HBOC)-201. Twenty-four livers declined for transplantation were transported by using static cold storage. Upon arrival, livers underwent NMP for 6 hours using pressure-controlled portal and arterial perfusion. A total of 12 livers were perfused using a solution based on RBCs and FFPs (historical cohort), 6 livers with HBOC-201 and FFPs, and another 6 livers with HBOC-201 and gelofusine, a gelatin-based colloid solution. Compared with RBC + FFP perfused livers, livers perfused with HBOC-201 had significantly higher hepatic adenosine triphosphate content, cumulative bile production, and portal and arterial flows. Biliary secretion of bicarbonate, bilirubin, bile salts, and phospholipids was similar in all 3 groups. The alanine aminotransferase concentration in perfusate was lower in the HBOC-201-perfused groups. In conclusion, NMP of human donor livers can be performed effectively using HBOC-201 and gelofusine, eliminating the need for human blood products. Perfusing livers with HBOC-201 is at least similar to perfusion with RBCs and FFP. Some of the biomarkers of liver function and injury even suggest a possible superiority of an HBOC-201-based perfusion solution and opens a perspective for further optimization of machine perfusion techniques. Liver Transplantation 24 528-538 2018 AASLD.
© 2017 The Authors. Liver Transplantation published by Wiley Periodicals, Inc. on behalf of American Association for the Study of Liver Diseases.
Figures





Comment in
-
Uploading cellular batteries: Caring for mitochondria is key.Liver Transpl. 2018 Apr;24(4):462-464. doi: 10.1002/lt.25036. Liver Transpl. 2018. PMID: 29460371 No abstract available.
-
Normothermic Machine Perfusion of Donor Livers Without the Need for Human Blood Products.Liver Transpl. 2018 Aug;24(8):1147-1148. doi: 10.1002/lt.25184. Liver Transpl. 2018. PMID: 29694701 No abstract available.
-
Reply.Liver Transpl. 2018 Aug;24(8):1149-1150. doi: 10.1002/lt.25183. Liver Transpl. 2018. PMID: 29694712 No abstract available.
References
-
- Barshes NR, Horwitz IB, Franzini L, Vierling JM, Goss JA. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant 2007;7:1265‐1270. - PubMed
-
- Matton AP, Porte RJ. Opportunities for scientific expansion of the deceased donor pool. Liver Transpl 2014;20(suppl 2):S5. - PubMed
-
- Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first‐in‐man) clinical trial. Am J Transplant 2016;16:1779‐1787. - PubMed
-
- Watson CJ, Kosmoliaptsis V, Randle LV, Russell NK, Griffiths WJ, Davies S, et al. Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant 2016;16:353‐357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

