Comparison of line probe assay to BACTEC MGIT 960 system for susceptibility testing of first and second-line anti-tuberculosis drugs in a referral laboratory in South Africa
- PMID: 29282012
- PMCID: PMC5745758
- DOI: 10.1186/s12879-017-2898-3
Comparison of line probe assay to BACTEC MGIT 960 system for susceptibility testing of first and second-line anti-tuberculosis drugs in a referral laboratory in South Africa
Abstract
Background: The incidence of multidrug-resistant tuberculosis (MDR-TB) is increasing and the emergence of extensively drug-resistant tuberculosis (XDR-TB) is a major challenge. Controlling resistance, reducing transmission and improving treatment outcomes in MDR/XDR-TB patients is reliant on susceptibility testing. Susceptibility testing using phenotypic methods is labour intensive and time-consuming. Alternative methods, such as molecular assays are easier to perform and have a rapid turn-around time. The World Health Organization (WHO) has endorsed the use of line probe assays (LPAs) for first and second line diagnostic screening of MDR/XDR-TB.
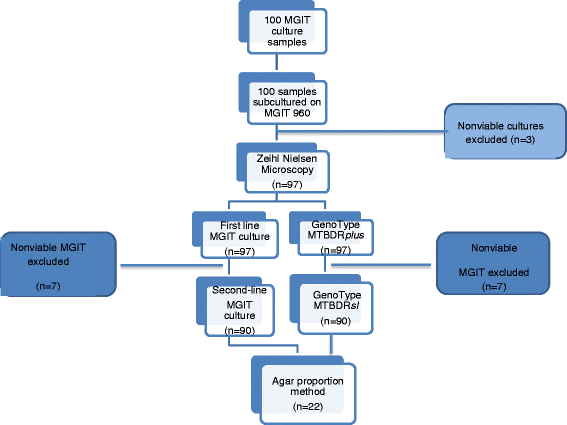
Methods: We compared the performance of LPAs to BACTEC MGIT 960 system for susceptibility testing of bacterial resistance to first-line drugs: rifampicin (RIF), isoniazid (INH), ethambutol (EMB), and second-line drugs ofloxacin (OFL) and kanamycin (KAN). One hundred (100) consecutive non-repeat Mycobacterium tuberculosis cultures, resistant to either INH or RIF or both, as identified by BACTEC MGIT 960 were tested. All isoniazid resistant cultures (n = 97) and RIF resistant cultures (n = 90) were processed with Genotype®MTBDRplus and Genotype®MTBDRsl line probe assays (LPAs). The agar proportion method was employed to further analyze discordant LPAs and the MGIT 960 isolates.
Results: The Genotype ®MTBDRplus (version 2) sensitivity, specificity, PPV and NPV from culture isolates were as follows: RIF, 100%, 87.9, 58.3% and 100%; INH, 100%, 94.4%, 93.5% and 100%. The sensitivity, specificity PPV and NPV for Genotype ® MTBDRsl (version 1 and 2) from culture isolates were as follows: EMB, 60.0%, 89.2%, 68.2% and 85.3%; OFL, 100%, 91.4%, 56.2% and 100%; KAN, 100%, 97.7%, 60.0% and 100%. Line probe assay showed an excellent agreement (k = 0.93) for INH susceptibility testing when compared to MGIT 960 system while there was good agreement (k = 0.6-0.7) between both methods for RIF, OFL, KAN testing and moderate agreement for EMB (k = 0.5). A high RIF mono-resistance (MGIT 960 33/97 and LPA 43/97) was observed.
Conclusion: LPAs are an efficient and reliable rapid molecular DST assay for rapid susceptibility screening of MDR and XDR-TB. Using LPAs in high MDR/XDR burden countries allows for appropriate and timely treatment, which will reduce transmission rates, morbidity and improve treatment outcomes in patients.
Keywords: Drug-resistance; Ethambutol; Isoniazid; Kanamycin; Line-probe assay; MGIT 960 system; Mycobacterium tuberculosis; Ofloxacin; Rifampicin.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was obtained from the Faculty of Health Science Research Ethics, University of Pretoria with protocol number 318/2013 and preceded experimental work.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization 2016 . Global Tuberculosis Report 2016. Geneva: World Health Organization; 2016.
-
- Heifets LB, Cangelosi GA. Drug susceptibility testing of Mycobacterium tuberculosis: a neglected problem at the turn of the century [State of the Art] Int J Tuberc Lung Dis. 1999;3:564–581. - PubMed
-
- Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Underst Stat. 1983;32:307–317.
-
- Nathavitharana RR, Cudahy PGT, Schumacher SG, Steingart KR, Pai M, Denkinger CM. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2017;49:1601075. doi: 10.1183/13993003.01075-2016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous