Azithromycin versus placebo for the treatment of HIV-associated chronic lung disease in children and adolescents (BREATHE trial): study protocol for a randomised controlled trial
- PMID: 29282143
- PMCID: PMC5745989
- DOI: 10.1186/s13063-017-2344-2
Azithromycin versus placebo for the treatment of HIV-associated chronic lung disease in children and adolescents (BREATHE trial): study protocol for a randomised controlled trial
Abstract
Background: Human immunodeficiency virus (HIV)-related chronic lung disease (CLD) among children is associated with substantial morbidity, despite antiretroviral therapy. This may be a consequence of repeated respiratory tract infections and/or dysregulated immune activation that accompanies HIV infection. Macrolides have anti-inflammatory and antimicrobial properties, and we hypothesised that azithromycin would reduce decline in lung function and morbidity through preventing respiratory tract infections and controlling systemic inflammation.
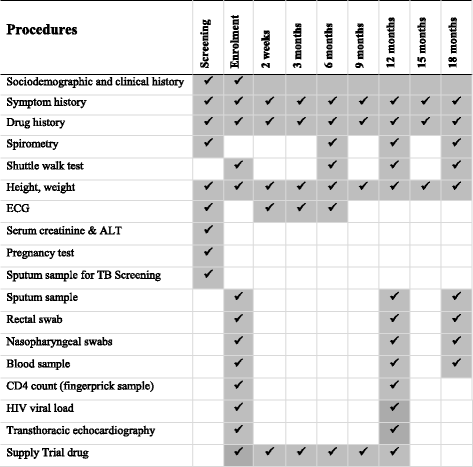
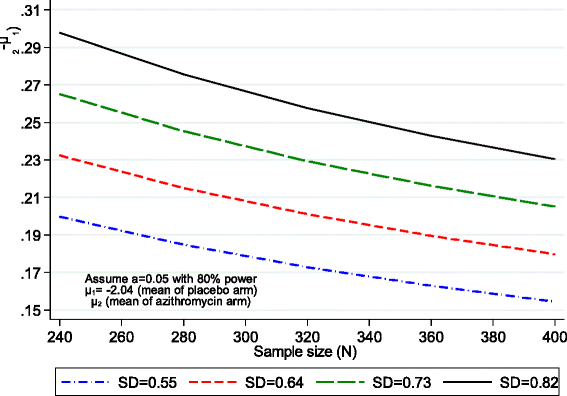
Methods/design: We are conducting a multicentre (Malawi and Zimbabwe), double-blind, randomised controlled trial of a 12-month course of weekly azithromycin versus placebo. The primary outcome is the mean change in forced expiratory volume in 1 second (FEV1) z-score at 12 months. Participants are followed up to 18 months to explore the durability of effect. Secondary outcomes are FEV1 z-score at 18 months, time to death, time to first acute respiratory exacerbation, number of exacerbations, number of hospitalisations, weight for age z-score at 12 and 18 months, number of adverse events, number of malaria episodes, number of bloodstream Salmonella typhi infections and number of gastroenteritis episodes. Participants will be followed up 3-monthly, and lung function will be assessed every 6 months. Laboratory substudies will be done to investigate the impact of azithromycin on systemic inflammation and on development of antimicrobial resistance as well as impact on the nasopharyngeal, lung and gut microbiome.
Discussion: The results of this trial will be of clinical relevance because there are no established guidelines on the treatment and management of HIV-associated CLD in children in sub-Saharan Africa, where 80% of the world's HIV-infected children live and where HIV-associated CLD is highly prevalent.
Trial registration: ClinicalTrials.gov, NCT02426112 . Registered on 21 April 2015.
Keywords: Africa; Azithromycin; Children; Chronic lung disease; FEV1; HIV; Obliterative bronchiolitis.
Conflict of interest statement
Ethics approval and consent to participate
Written informed consent by the participants’ guardians and assent will be obtained from participants aged younger than 18 years of age using an age-appropriate assent form before enrolment. Participants older than 18 years of age will be able to consent independently. The trial was approved by the London School of Hygiene and Tropical Medicine Ethics Committee (reference 8818) on 3 June 2015; by the Harare Central Hospital Ethics Committee on 5 October 2015; by the Medical Research Council of Zimbabwe (reference MRCZ/A/1946) on 24 May 2016; by the College of Medicine Research Ethics Committee Malawi (reference P.04/15/1719) on 7 August 2015; by the Medical Committee for Medical and Health Research Ethics, Northern Norway (reference 2015/1650) on 18 September 2015; and by the University of Cape Town Ethics Committee (reference 754/2015) on 30 June 2016. The University of Oxford did not require additional approval. Approval for conducting the clinical trial and for importation of the study investigational products was obtained from the Medicines Control Authority of Zimbabwe (26 January 2016; reference B/279/5/14/2016) and from the Pharmacy, Medicines and Poisons Board Malawi (9 March 2016; reference PMPB/CTRC/III/76).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Rylance J, Meghji J, Miller RF, Ferrand RA. Global considerations in human immunodeficiency virus-associated respiratory disease. Semin Respir Crit Care Med. 2016;37(2):166–80. doi:10.1055/s-0036-1572555. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous