Safety and efficacy of inspiratory muscle training for preventing adverse outcomes in patients at risk of prolonged hospitalisation
- PMID: 29282152
- PMCID: PMC5745884
- DOI: 10.1186/s13063-017-2372-y
Safety and efficacy of inspiratory muscle training for preventing adverse outcomes in patients at risk of prolonged hospitalisation
Abstract
Background: The early institution of inspiratory muscle training on hospitalised patients with no established respiratory deficits could prevent in-hospital adverse outcomes that are directly or indirectly associated to the loss of respiratory muscle mass inherent to a prolonged hospital stay. The objective of the clinical trial is to assess the impact of inspiratory muscle training on hospital inpatient complications.
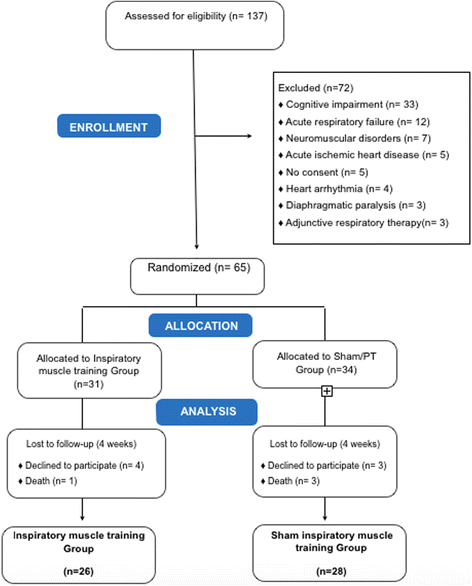
Methods: This is a double-blind randomised controlled trial. Subjects in the intervention group underwent an inspiratory muscle training loaded with 50% maximum inspiratory pressure twice daily for 4 weeks from study enrolment. Patients were randomly assigned to an inspiratory muscle training group or a sham inspiratory muscle training group. All patients received conventional physiotherapy interventions. Baseline and post-intervention respiratory and peripheral muscle strength, functionality (performance of activities of daily living), length of hospital stay, and death were evaluated. Clinical outcomes were assessed until hospital discharge. This study was approved by the Institutional Hospital Ethics Committee (03/2014).
Results: Thirty-one patients assigned to the inspiratory muscle training group and 34 to the sham inspiratory muscle training group were analysed. Patients in the inspiratory muscle training group had a shorter mean length of hospital stay (35.3 ± 2.7 vs. 41.8 ± 3.5 days, p < 0.01) and a lower risk of endotracheal intubation (relative risk (RR) = 0.36; 95% confidence interval (CI) 0.27-0.97; p = 0.03) as well as muscle weakness (RR = 0.36; 95% CI 0.19-0.98; p = 0.02) and mortality (RR = 0.23; 95% CI 0.2-0.94; p = 0.04). The risk of adverse events did not differ significantly between groups.
Conclusion: Inspiratory muscle training was a protective factor against endotracheal intubation, muscle weakness, and mortality.
Trial registration: ClinicalTrials.gov, ID: NCT02459444 . Registered on 19 May 2015.
Keywords: Functionality; Hospitalisation; Length of stay; Mortality; Muscle weakness; Respiratory muscles.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
We declare that no part of the submitted work has been published or is under consideration for publication elsewhere, and there is no financial or other relationship that might lead to a conflict of interest. All co-authors have seen and approved the study submitted. This study was approved by the Ethics Committee of the Roberto Santos General Hospital (approval reference number 03/2014). All patients or their legal guardians signed the consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–20. doi: 10.1164/rccm.201312-2257OC. - DOI - PubMed
-
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2005;364(14):1209–23. - PubMed
-
- Hudson MB, Smuder AJ, Nelson WB, Wiggs MP, Shimkus KL, Fluckey JD, et al. Partial support ventilation and mitochondrial-targeted antioxidants protect against ventilator-induced decreases in diaphragm muscle protein synthesis. PLoS One. 2015;10(9):1–17. doi: 10.1371/journal.pone.0137693. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical