The bright and the dark side of human antibody responses to flaviviruses: lessons for vaccine design
- PMID: 29282215
- PMCID: PMC5797954
- DOI: 10.15252/embr.201745302
The bright and the dark side of human antibody responses to flaviviruses: lessons for vaccine design
Abstract
Zika and dengue viruses belong to the Flavivirus genus, a close group of antigenically related viruses that cause significant arthropod-transmitted diseases throughout the globe. Although infection by a given flavivirus is thought to confer lifelong protection, some of the patient's antibodies cross-react with other flaviviruses without cross-neutralizing. The original antigenic sin phenomenon may amplify such antibodies upon subsequent heterologous flavivirus infection, potentially aggravating disease by antibody-dependent enhancement (ADE). The most striking example is provided by the four different dengue viruses, where infection by one serotype appears to predispose to more severe disease upon infection by a second one. A similar effect was postulated for sequential infections with Zika and dengue viruses. In this review, we analyze the molecular determinants of the dual antibody response to flavivirus infection or vaccination in humans. We highlight the role of conserved partially cryptic epitopes giving rise to cross-reacting and poorly neutralizing, ADE-prone antibodies. We end by proposing a strategy for developing an epitope-focused vaccine approach to avoid eliciting undesirable antibodies while focusing the immune system on producing protective antibodies only.
Keywords: antibody neutralization; antibody‐dependent enhancement; flavivirus structure; particle heterogeneity; vaccine design.
© 2017 Institut Pasteur. Published under the terms of the CC BY NC ND 4.0 license.
Figures
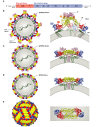
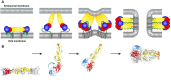


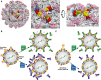
References
-
- WHO (2017) Yellow fever in Africa and the Americas, 2016. Wkly Epidemiol Rec 92: 442–452 - PubMed
-
- ECDC (2017) Rapid risk assessment: outbreak of yellow fever in Brazil. First update, 13 April 2017. Stockholm: ECDC;
-
- Wilder‐Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW (2017) Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis 17: e101–e106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

