Moesin is involved in polarity maintenance and cortical remodeling during asymmetric cell division
- PMID: 29282284
- PMCID: PMC6014166
- DOI: 10.1091/mbc.E17-05-0294
Moesin is involved in polarity maintenance and cortical remodeling during asymmetric cell division
Abstract
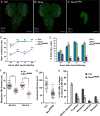
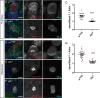
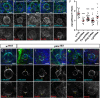
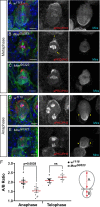
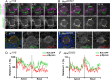
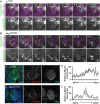
An intact actomyosin network is essential for anchoring polarity proteins to the cell cortex and maintaining cell size asymmetry during asymmetric cell division of Drosophila neuroblasts (NBs). However, the mechanisms that control changes in actomyosin dynamics during asymmetric cell division remain unclear. We find that the actin-binding protein, Moesin, is essential for NB proliferation and mitotic progression in the developing brain. During metaphase, phosphorylated Moesin (p-Moesin) is enriched at the apical cortex, and loss of Moesin leads to defects in apical polarity maintenance and cortical stability. This asymmetric distribution of p-Moesin is determined by components of the apical polarity complex and Slik kinase. During later stages of mitosis, p-Moesin localization shifts more basally, contributing to asymmetric cortical extension and myosin basal furrow positioning. Our findings reveal Moesin as a novel apical polarity protein that drives cortical remodeling of dividing NBs, which is essential for polarity maintenance and initial establishment of cell size asymmetry.
© 2018 Abeysundara et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



References
-
- Albertson R, Chabu C, Sheehan A, Doe CQ. Scribble protein domain mapping reveals a multistep localization mechanism and domains necessary for establishing cortical polarity. J Cell Sci. 2004:6061–6070. - PubMed
-
- Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003:166–170. - PubMed
-
- Bardet PL, Guirao B, Paoletti C, Serman F, Leopold V, Bosveld F, Goya Y, Mirouse V, Graner F, Bellaiche Y. PTEN controls junction lengthening and stability during cell rearrangement in epithelial tissue. Dev Cell. 2013:534–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

