CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP
- PMID: 29282336
- PMCID: PMC5798651
- DOI: 10.1212/WNL.0000000000004859
CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP
Abstract
Objective: To determine the ability of CSF biomarkers to predict disease progression in progressive supranuclear palsy (PSP).
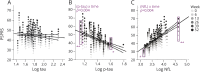
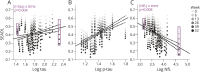
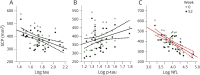
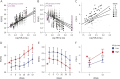
Methods: We compared the ability of baseline CSF β-amyloid1-42, tau, phosphorylated tau 181 (p-tau), and neurofilament light chain (NfL) concentrations, measured by INNO-BIA AlzBio3 or ELISA, to predict 52-week changes in clinical (PSP Rating Scale [PSPRS] and Schwab and England Activities of Daily Living [SEADL]), neuropsychological, and regional brain volumes on MRI using linear mixed effects models controlled for age, sex, and baseline disease severity, and Fisher F density curves to compare effect sizes in 50 patients with PSP. Similar analyses were done using plasma NfL measured by single molecule arrays in 141 patients.
Results: Higher CSF NfL concentration predicted more rapid decline (biomarker × time interaction) over 52 weeks in PSPRS (p = 0.004, false discovery rate-corrected) and SEADL (p = 0.008), whereas lower baseline CSF p-tau predicted faster decline on PSPRS (p = 0.004). Higher CSF tau concentrations predicted faster decline by SEADL (p = 0.004). The CSF NfL/p-tau ratio was superior for predicting change in PSPRS, compared to p-tau (p = 0.003) or NfL (p = 0.001) alone. Higher NfL concentrations in CSF or blood were associated with greater superior cerebellar peduncle atrophy (fixed effect, p ≤ 0.029 and 0.008, respectively).
Conclusions: Both CSF p-tau and NfL correlate with disease severity and rate of disease progression in PSP. The inverse correlation of p-tau with disease severity suggests a potentially different mechanism of tau pathology in PSP as compared to Alzheimer disease.
Copyright © 2017 American Academy of Neurology.
Figures
References
-
- Mattsson N, Lonneborg A, Boccardi M, Blennow K, Hansson O, Geneva Task Force for the Roadmap of Alzheimer's Biomarkers. Clinical validity of cerebrospinal fluid Abeta42, tau, and phospho-tau as biomarkers for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging 2017;52:196–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
