Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial
- PMID: 29282469
- PMCID: PMC5838571
- DOI: 10.1001/jamapsychiatry.2017.3739
Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial
Abstract
Importance: Approximately one-third of patients with major depressive disorder (MDD) do not respond to available antidepressants.
Objective: To assess the efficacy, safety, and dose-response of intranasal esketamine hydrochloride in patients with treatment-resistant depression (TRD).
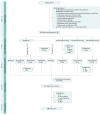
Design, setting, and participants: This phase 2, double-blind, doubly randomized, delayed-start, placebo-controlled study was conducted in multiple outpatient referral centers from January 28, 2014, to September 25, 2015. The study consisted of 4 phases: (1) screening, (2) double-blind treatment (days 1-15), composed of two 1-week periods, (3) optional open-label treatment (days 15-74), and (4) posttreatment follow-up (8 weeks). One hundred twenty-six adults with a DSM-IV-TR diagnosis of MDD and history of inadequate response to 2 or more antidepressants (ie, TRD) were screened, 67 were randomized, and 60 completed both double-blind periods. Intent-to-treat analysis was used in evaluation of the findings.
Interventions: In period 1, participants were randomized (3:1:1:1) to placebo (n = 33), esketamine 28 mg (n = 11), 56 mg (n = 11), or 84 mg (n = 12) twice weekly. In period 2, 28 placebo-treated participants with moderate-to-severe symptoms were rerandomized (1:1:1:1) to 1 of the 4 treatment arms; those with mild symptoms continued receiving placebo. Participants continued their existing antidepressant treatment during the study. During the open-label phase, dosing frequency was reduced from twice weekly to weekly, and then to every 2 weeks.
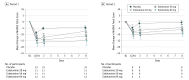
Main outcomes and measures: The primary efficacy end point was change from baseline to day 8 (each period) in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score.
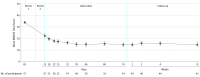
Results: Sixty-seven participants (38 women, mean [SD] age, 44.7 [10.0] years) were included in the efficacy and safety analyses. Change (least squares mean [SE] difference vs placebo) in MADRS total score (both periods combined) in all 3 esketamine groups was superior to placebo (esketamine 28 mg: -4.2 [2.09], P = .02; 56 mg: -6.3 [2.07], P = .001; 84 mg: -9.0 [2.13], P < .001), with a significant ascending dose-response relationship (P < .001). Improvement in depressive symptoms appeared to be sustained (-7.2 [1.84]) despite reduced dosing frequency in the open-label phase. Three of 56 (5%) esketamine-treated participants during the double-blind phase vs none receiving placebo and 1 of 57 participants (2%) during the open-label phase had adverse events that led to study discontinuation (1 event each of syncope, headache, dissociative syndrome, and ectopic pregnancy).
Conclusions and relevance: In this first clinical study to date of intranasal esketamine for TRD, antidepressant effect was rapid in onset and dose related. Response appeared to persist for more than 2 months with a lower dosing frequency. Results support further investigation in larger trials.
Trial registration: clinicaltrials.gov identifier: NCT01998958.
Conflict of interest statement
Figures
Comment in
-
The Promise of Intranasal Esketamine as a Novel and Effective Antidepressant.JAMA Psychiatry. 2018 Feb 1;75(2):123-124. doi: 10.1001/jamapsychiatry.2017.3738. JAMA Psychiatry. 2018. PMID: 29282452 No abstract available.
-
Adjunctive Intranasal Esketamine in Treatment-Resistant Depression-Reply.JAMA Psychiatry. 2018 Jun 1;75(6):654-655. doi: 10.1001/jamapsychiatry.2018.0430. JAMA Psychiatry. 2018. PMID: 29800990 No abstract available.
-
Adjunctive Intranasal Esketamine in Treatment-Resistant Depression.JAMA Psychiatry. 2018 Jun 1;75(6):654. doi: 10.1001/jamapsychiatry.2018.0690. JAMA Psychiatry. 2018. PMID: 29801086 No abstract available.
References
-
- Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. - PMC - PubMed
-
- Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients: results from the Medical Outcomes Study. JAMA. 1989;262(7):914-919. - PubMed
-
- Daly EJ, Trivedi MH, Wisniewski SR, et al. Health-related quality of life in depression: a STAR*D report. Ann Clin Psychiatry. 2010;22(1):43-55. - PubMed
-
- de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619-630. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

