Efficacy and Safety of Transcranial Direct Current Stimulation as an Add-on Treatment for Bipolar Depression: A Randomized Clinical Trial
- PMID: 29282470
- PMCID: PMC5838572
- DOI: 10.1001/jamapsychiatry.2017.4040
Efficacy and Safety of Transcranial Direct Current Stimulation as an Add-on Treatment for Bipolar Depression: A Randomized Clinical Trial
Abstract
Importance: More effective, tolerable interventions for bipolar depression treatment are needed. Transcranial direct current stimulation (tDCS) is a novel therapeutic modality with few severe adverse events that showed promising results for unipolar depression.
Objective: To determine the efficacy and safety of tDCS as an add-on treatment for bipolar depression.
Design, setting, and participants: A randomized, sham-controlled, double-blind trial (the Bipolar Depression Electrical Treatment Trial [BETTER]) was conducted from July 1, 2014, to March 30, 2016, at an outpatient, single-center academic setting. Participants included 59 adults with type I or II bipolar disorder in a major depressive episode and receiving a stable pharmacologic regimen with 17-item Hamilton Depression Rating Scale (HDRS-17) scores higher than 17. Data were analyzed in the intention-to-treat sample.
Interventions: Ten daily 30-minute, 2-mA, anodal-left and cathodal-right prefrontal sessions of active or sham tDCS on weekdays and then 1 session every fortnight until week 6.
Main outcomes and measures: Change in HDRS-17 scores at week 6.
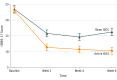
Results: Fifty-nine patients (40 [68%] women), with a mean (SD) age of 45.9 (12) years participated; 36 (61%) with bipolar I and 23 (39%) with bipolar II disorder were randomized and 52 finished the trial. In the intention-to-treat analysis, patients in the active tDCS condition showed significantly superior improvement compared with those receiving sham (βint = -1.68; number needed to treat, 5.8; 95% CI, 3.3-25.8; P = .01). Cumulative response rates were higher in the active vs sham groups (67.6% vs 30.4%; number needed to treat, 2.69; 95% CI, 1.84-4.99; P = .01), but not remission rates (37.4% vs 19.1%; number needed to treat, 5.46; 95% CI, 3.38-14.2; P = .18). Adverse events, including treatment-emergent affective switches, were similar between groups, except for localized skin redness that was higher in the active group (54% vs 19%; P = .01).
Conclusions and relevance: In this trial, tDCS was an effective, safe, and tolerable add-on intervention for this small bipolar depression sample. Further trials should examine tDCS efficacy in a larger sample.
Trial registration: clinicaltrials.gov Identifier: NCT02152878.
Conflict of interest statement
Figures


Comment in
-
Commentary: Efficacy and Safety of Transcranial Direct Current Stimulation as an Add-on Treatment for Bipolar Depression: A Randomized Clinical Trial.Front Hum Neurosci. 2018 Dec 3;12:480. doi: 10.3389/fnhum.2018.00480. eCollection 2018. Front Hum Neurosci. 2018. PMID: 30574077 Free PMC article. No abstract available.
References
-
- Ferrari AJ, Stockings E, Khoo JP, et al. . The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18(5):440-450. - PubMed
-
- Judd LL, Akiskal HS, Schettler PJ, et al. . The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537. - PubMed
-
- Schoeyen HK, Kessler U, Andreassen OA, et al. . Treatment-resistant bipolar depression: a randomized controlled trial of electroconvulsive therapy versus algorithm-based pharmacological treatment. Am J Psychiatry. 2015;172(1):41-51. - PubMed
-
- Brunoni AR, Chaimani A, Moffa AH, et al. . Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry. 2017;74(2):143-152. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

