Human T cell responses to Dengue and Zika virus infection compared to Dengue/Zika coinfection
- PMID: 29282904
- PMCID: PMC5946158
- DOI: 10.1002/iid3.203
Human T cell responses to Dengue and Zika virus infection compared to Dengue/Zika coinfection
Abstract
Introduction: Zika virus (ZIKV) and dengue virus (DENV) co-circulated during latest outbreaks in Brazil, hence, it is important to evaluate the host cross-reactive immune responses to these viruses. So far, little is known about human T cell responses to ZIKV and no reports detail adaptive immune responses during DENV/ZIKV coinfection.
Methods: Here, we studied T cells responses in well-characterized groups of DENV, ZIKV, or DENV/ZIKV infected patients and DENV-exposed healthy donors. We evaluated chemokine receptors expression and single/multifunctional frequencies of IFNγ, TNF, and IL2-producing T cells during these infections. Even without antigenic stimulation, it was possible to detect chemokine receptors and IFNγ, TNF, and IL2-producing T cells from all individuals by flow cytometry. Additionally, PBMCs' IFNγ response to DENV NS1 protein and to polyclonal stimuli was evaluated by ELISPOT.
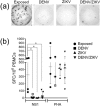
Results: DENV and ZIKV infections and DENV/ZIKV coinfections similarly induced expression of CCR5, CX3CR1, and CXCR3 on CD4 and CD8 T cells. DENV/ZIKV coinfection decreased the ability of CD4+ T cells to produce IFNγ+ , TNF+ , TNF + IFNγ+ , and TNF + IL2+ , compared to DENV and ZIKV infections. A higher magnitude of IFNγ response to DENV NS1 was found in donors with a history of dengue infection, however, a hyporesponsiveness was found in acute DENV, ZIKV, or DENV/ZIKV infected patients, even previously infected with DENV.
Conclusion: Therefore, we emphasize the potential impact of coinfection on the immune response from human hosts, mainly in areas where DENV and ZIKV cocirculate.
Keywords: Dengue; Zika: T lymphocytes.
© 2017 The Authors. Immunity, Inflammation and Disease Published by John Wiley & Sons Ltd.
Figures


References
-
- Kuno, G. , and G‐JJ C.. 2007. Full‐length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch. Virol. 152:687–696. - PubMed
-
- Guzmán, M. G. , and Kourí G.. 2002. Dengue: an update. Lancet Infect. Dis. 2:33–42. - PubMed
-
- WHO. Dengue Fact Sheet. http://www.searo.who.int/vector_borne_tropical_diseases/data/data_factsh... (2017, accessed 24 February 2017).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

