Photo-responsive Bioactive Surfaces Based on Cucurbit[8]uril-Mediated Host-Guest Interactions of Arylazopyrazoles
- PMID: 29283194
- PMCID: PMC5814888
- DOI: 10.1002/chem.201705426
Photo-responsive Bioactive Surfaces Based on Cucurbit[8]uril-Mediated Host-Guest Interactions of Arylazopyrazoles
Abstract
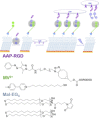
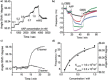
A photoswitchable arylazopyrazole (AAP) derivative binds with cucurbit[8]uril (CB[8]) and methylviologen (MV2+ ) to form a 1:1:1 heteroternary host-guest complex with a binding constant of Ka =2×103 m-1 . The excellent photoswitching properties of AAP are preserved in the inclusion complex. Irradiation with light of a wavelength of 365 and 520 nm leads to quantitative E- to Z- isomerization and vice versa, respectively. Formation of the Z-isomer leads to dissociation of the complex as evidenced using 1 H NMR spectroscopy. AAP derivatives are then used to immobilize bioactive molecules and photorelease them on demand. When Arg-Gly-Asp-AAP (AAP-RGD) peptides are attached to surface bound CB[8]/MV2+ complexes, cells adhere and can be released upon irradiation. The heteroternary host-guest system offers highly reversible binding properties due to efficient photoswitching and these properties are attractive for designing smart surfaces.
Keywords: arylazopyrazoles; cucurbit[8]uril; host-guest systems; photo-responsive; stimuli-responsive.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brinkmann J., Cavatorta E., Sankaran S., Schmidt B., van Weerd J., Jonkheijm P., Chem. Soc. Rev. 2014, 43, 4449. - PubMed
-
- Yang H., Yuan B., Zhang X., Scherman O. A., Acc. Chem. Res. 2014, 47, 2106. - PubMed
-
- Chilkoti A., Hubbell J. A., MRS Bull. 2005, 30, 175.
-
- Love J. C., Estroff L. A., Kriebel J. K., Nuzzo R. G., Whitesides G. M., Chem. Rev. 2005, 105, 1103. - PubMed
-
- Feringa B. L., van Delden R. A., Koumura N., Geertsema E. M., Chem. Rev. 2000, 100, 1789. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

