CuH-Catalyzed Asymmetric Reduction of α,β-Unsaturated Carboxylic Acids to β-Chiral Aldehydes
- PMID: 29283578
- PMCID: PMC5800953
- DOI: 10.1021/jacs.7b12260
CuH-Catalyzed Asymmetric Reduction of α,β-Unsaturated Carboxylic Acids to β-Chiral Aldehydes
Abstract
The copper hydride (CuH)-catalyzed enantioselective reduction of α,β-unsaturated carboxylic acids to saturated aldehydes is reported. This protocol provides a new method to access a variety of β-chiral aldehydes in good yields, with high levels of enantioselectivity and broad functional group tolerance. A reaction pathway involving a ketene intermediate is proposed based on preliminary mechanistic studies and density functional theory calculations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
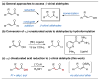

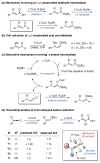

References
-
- Brossi A, Grethe G, Teltel S, Wildman WC, Bailey DT. J Org Chem. 1970;35:1100.
- Welch WM, Kraska AR, Sarges R, Koe BK. J Med Chem. 1984;27:1508. - PubMed
- Hyttel J, Larsen JJ. J Neurochem. 1985;44:1615. - PubMed
- Nilvebrant L, Andersson KE, Gillberg PG, Stahl M, Sparf B. Eur J Pharmacol. 1997;327:195. - PubMed
- Andersson PG, Schink HE, Österlund K. J Org Chem. 1998;63:8067.
- Selenski C, Pettus TRR. J Org Chem. 2004;69:9196. - PubMed
-
-
For reviews and selected examples, see: Zheng C, You SL. Chem Soc Rev. 2012;41:2498.Phillips AMF, Pombeiro AJL. Org Biomol Chem. 2017;15:2307.Ouellet SG, Tuttle JB, MacMillan DWC. J Am Chem Soc. 2005;127:32.Yang JW, Hechavarria Fonseca MT, List B. Angew Chem, Int Ed. 2004;43:6660.Mayer S, List B. Angew Chem, Int Ed. 2006;45:4193.
-
-
-
For selected examples of transition-metal-catalyzed asymmetric conjugated addition reactions, see: Itooka R, Iguchi Y, Miyaura N. J Org Chem. 2003;68:6000.Paquin JF, Defieber C, Stephenson CRJ, Carreira EM. J Am Chem Soc. 2005;127:10850.Hayashi T, Tokunaga N, Okamoto K, Shintani R. Chem Lett. 2005;34:1480.Bräse S, Höfener S. Angew Chem, Int Ed. 2005;44:7879.Nishimura T, Sawano T, Hayashi T. Angew Chem, Int Ed. 2009;48:8057.Ibrahem I, Ma G, Afewerki S, Córdova A. Angew Chem, Int Ed. 2013;52:878.
-
-
-
For selected examples of organocatalysis catalyzed asymmetric conjugated addition, see: Paras NA, MacMillan DWC. J Am Chem Soc. 2002;124:7894.Ooi T, Doda K, Maruoka K. J Am Chem Soc. 2003;125:9022.Wang W, Li H, Wang J. Org Lett. 2005;7:1637.Wu F, Li H, Hong R, Khan J, Liu X, Deng L. Angew Chem, Int Ed. 2006;45:4301.Brandau S, Landa A, Franzén J, Marigo M, Jørgensen KA. Angew Chem, Int Ed. 2006;45:4305.Chen YK, Yoshida M, MacMillan DWC. J Am Chem Soc. 2006;128:9328.Lee S, MacMillan DWC. J Am Chem Soc. 2007;129:15438.Akagawa K, Kudo K. Angew Chem, Int Ed. 2012;51:12786.
-
-
-
For reviews and selected examples, see: Cahard D, Gaillard S, Renaud JL. Tetrahedron Lett. 2015;56:6159.Li H, Mazet C. Acc Chem Res. 2016;49:1232.Tanaka K, Qiao S, Tobisu M, Lo MMC, Fu GC. J Am Chem Soc. 2000;122:9870.Doppiu A, Salzer A. Eur J Inorg Chem. 2004:2244.Mantilli L, Gérard D, Torche S, Besnard C, Mazet C. Angew Chem, Int Ed. 2009;48:5413.Quintard A, Alexakis A, Mazet C. Angew Chem, Int Ed. 2011;50:2354.Wu R, Beauchamps MG, Laquidara JM, Sowa JR. Angew Chem, Int Ed. 2012;51:2106.Arai N, Sato K, Azuma K, Ohkuma T. Angew Chem, Int Ed. 2012;51:12786.Larionov E, Lin L, Guénée L, Mazet C. J Am Chem Soc. 2014;136:16882.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

