Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma
- PMID: 29284010
- PMCID: PMC5746232
- DOI: 10.1371/journal.pone.0189848
Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma
Abstract
Background: Colorectal cancers (CRCs) expressing programmed death ligand 1 (PD-L1) have poor prognosis. In the multicohort KEYNOTE-028 trial, the anti-PD-1 antibody pembrolizumab was evaluated in 20 PD-L1-positive advanced solid tumors. Herein, we report results for the advanced CRC cohort.
Methods: Patients with advanced, treatment-resistant PD-L1-positive carcinoma of the colon or rectum were enrolled, regardless of microsatellite instability (MSI) status. Pembrolizumab 10 mg/kg was administered every 2 weeks for up to 2 years or until disease progression/unacceptable toxicity. Response was assessed every 8 weeks for the first 6 months and every 12 weeks thereafter. Primary end points were safety and overall response rate by investigator review per Response Evaluation Criteria in Solid Tumors version 1.1. Data cutoff was June 20, 2016.
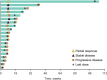
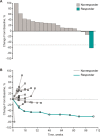
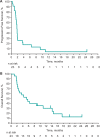
Results: Of 137 patients with CRC and samples evaluable for PD-L1 expression, 33 (24%) had PD-L1-positive tumors, of which 23 were enrolled. Median follow-up was 5.3 months, and 8 patients (35%) reported treatment-related adverse events (AEs), most commonly fatigue (n = 3, 13%), stomatitis (n = 2, 9%), and asthenia (n = 2, 9%). One patient (4%) experienced grade 4 treatment-related increased blood bilirubin. No grade 3 AEs, discontinuations, or deaths were attributed to treatment. Most patients (n = 15, 65%) experienced progressive disease. One partial response occurred in a patient (4%) with MSI-high CRC.
Conclusion: Pembrolizumab demonstrated a favorable safety profile in advanced PD-L1-positive CRC. Antitumor activity was observed in a single patient with MSI-high CRC, warranting further evaluation in this patient population. (Clinicaltrials.gov registration: NCT02054806).
Conflict of interest statement
Figures

References
-
- American Cancer Society: Colorectal Cancer Facts and Figures 2014–2016. http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.... Accessed March 7, 2017.
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. doi: 10.3322/caac.21332 - DOI - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. doi: 10.3322/caac.21262 - DOI - PubMed
-
- National Cancer Institute: SEER Stat Fact Sheets: Colon and Rectum Cancer 2016. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed June 13, 2016
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

