Calcium-sensing receptor-mediated L-tryptophan-induced secretion of cholecystokinin and glucose-dependent insulinotropic peptide in swine duodenum
- PMID: 29284209
- PMCID: PMC5879066
- DOI: 10.4142/jvs.2018.19.2.179
Calcium-sensing receptor-mediated L-tryptophan-induced secretion of cholecystokinin and glucose-dependent insulinotropic peptide in swine duodenum
Abstract
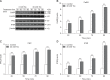
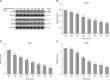
This study aimed to elucidate the effect of tryptophan (Trp) on gut hormone secretion as well as the roles of the calcium-sensing receptor (CaSR) and its downstream signaling pathway in gut hormone secretion by assessing swine duodenal perfusion in vitro. Swine duodenum was perfused with Krebs-Henseleit buffer as a basal solution. Various concentrations (0, 10, and 20 mM) of Trp were applied to investigate its effect on gut hormone secretion. A CaSR antagonist was used to detect the involvement of CaSR and its signal molecules. The 20 mM Trp concentration promoted the secretion of cholecystokinin (CCK) and glucose-dependent insulinotropic peptide (GIP), elevated the mRNA level of CaSR, and upregulated the protein levels of CaSR, protein kinase C (PKC), and inositol trisphosphate receptor (IP3R). However, NPS 2143, an inhibitor of CaSR, attenuated the CCK and GIP release, reduced the mRNA level of CaSR, and decreased the protein levels of CaSR, PKC, and IP3R with 20 mM Trp perfusion. The results indicate that CCK and GIP secretion can be induced by Trp in swine duodenum in vitro, and the effect is mediated by CaSR and its downstream signal molecules PKC and IP3R.
Keywords: calcium-sensing receptor; gut hormone; signaling pathway; swine; tryptophan.
Conflict of interest statement
Figures


References
-
- Badger TM. Perifusion of anterior pituitary cells: release of gonadotropins and somatotropins. Methods Enzymol. 1986;124:79–90. - PubMed
-
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. - PubMed
-
- Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol. 2006;291:G753–G761. - PubMed
-
- Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr. 2002;56:1072–1080. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

