Adaptation of the WHO maternal near miss tool for use in sub-Saharan Africa: an International Delphi study
- PMID: 29284433
- PMCID: PMC5747119
- DOI: 10.1186/s12884-017-1640-x
Adaptation of the WHO maternal near miss tool for use in sub-Saharan Africa: an International Delphi study
Abstract
Background: Assessments of maternal near miss (MNM) are increasingly used in addition to those of maternal mortality measures. The World Health Organization (WHO) has introduced an MNM tool in 2009, but this tool was previously found to be of limited applicability in several low-resource settings. The aim of this study was to identify adaptations to enhance applicability of the WHO MNM tool in sub-Saharan Africa.
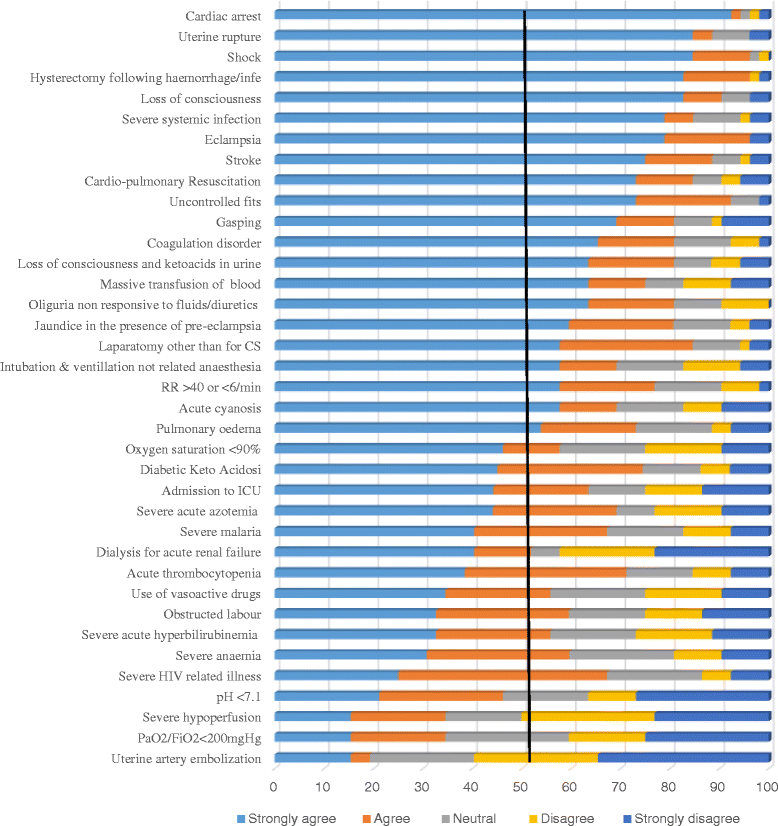
Methods: Using a Delphi consensus methodology, existing MNM tools were rated for applicability in sub-Saharan Africa over a series of three rounds. Maternal health experts from sub-Saharan Africa or with considerable knowledge of the context first rated importance of WHO MNM parameters using Likert scales, and were asked to suggest additional parameters. This was followed by two confirmation rounds. Parameters accepted by at least 70% of the panel members were accepted for use in the region.
Results: Of 58 experts who participated from study onset, 47 (81%) completed all three rounds. Out of the 25 WHO MNM parameters, all 11 clinical, four out of eight laboratory, and four out of six management-based parameters were accepted, while six parameters (PaO2/FiO2 < 200 mmHg, bilirubin >100 μmol/l or >6.0 mg/dl, pH <7.1, lactate >5 μmol/l, dialysis for acute renal failure and use of continuous vasoactive drugs) were deemed to not be applicable. An additional eight parameters (uterine rupture, sepsis/severe systemic infection, eclampsia, laparotomy other than caesarean section, pulmonary edema, severe malaria, severe complications of abortions and severe pre-eclampsia with ICU admission) were suggested for inclusion into an adapted sub-Saharan African MNM tool.
Conclusions: All WHO clinical criteria were accepted for use in the region. Only few of the laboratory- and management based were rated applicable. This study brought forward important suggestions for adaptations in the WHO MNM criteria to enhance its applicability in sub-Saharan Africa and possibly other low-resource settings.
Keywords: Delphi; Global health; Maternal near miss; Severe maternal morbidity; Sub–Saharan Africa.
Conflict of interest statement
Ethics approval and consent to participate
This study was conducted as part of a PhD study on maternal near miss and maternal mortality in Ethiopia which was approved by the institutional health research ethics review committee of Haramaya University in Ethiopia (Ref No: C/A/R/D/01/1681/16). This study does not include any patient-related or otherwise sensitive information. Informed consent was requested from all experts and completing online questionnaire was considered as consent to participation.
Consent for publication
Not applicable
Competing interests
JvR is section editor for BMC Pregnancy and Childbirth. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization . Beyond the numbers: reviewing maternal deaths and complications to make pregnancy safer. Geneva: World Health Organization; 2004.
-
- World Health Organization . Evaluating the quality of Care for Severe Pregnancy Complications: the who near-miss approach for maternal health. Geneva: World Health Organization; 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical