Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT)
- PMID: 29284438
- PMCID: PMC5745644
- DOI: 10.1186/s12888-017-1571-0
Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT)
Abstract
Background: People with psychosis have a reduced life expectancy of 10-20 years, largely due to cardiovascular disease. This trial aimed to determine the effectiveness of a modular health promotion intervention (IMPaCT Therapy) in improving health and reducing cardiovascular risk in psychosis.
Methods: A multicentre, two arm, parallel cluster RCT was conducted across five UK mental health NHS trusts. Community care coordinators (CC) were randomly assigned to training and supervision in delivering IMPaCT Therapy or treatment as usual (TAU) to current patients with psychosis (cluster). The primary outcome was the physical and mental health subscales of the Short form-36 (SF-36) questionnaire.
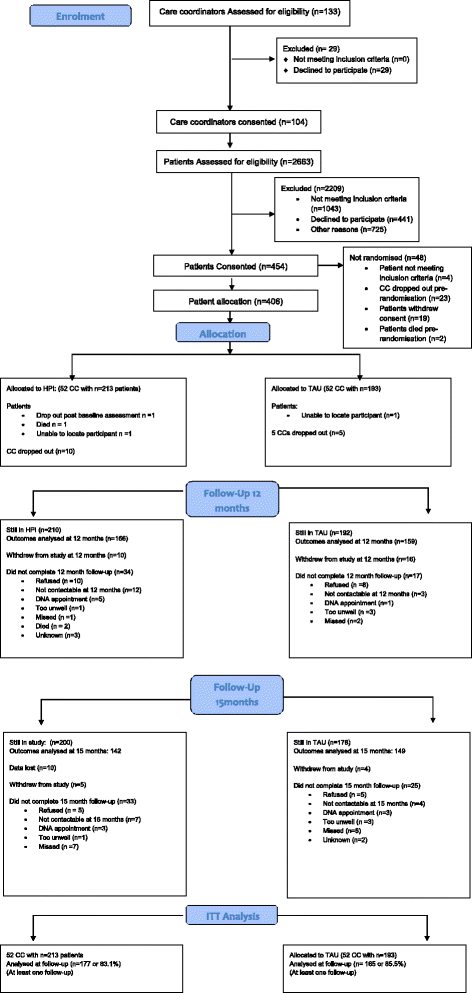
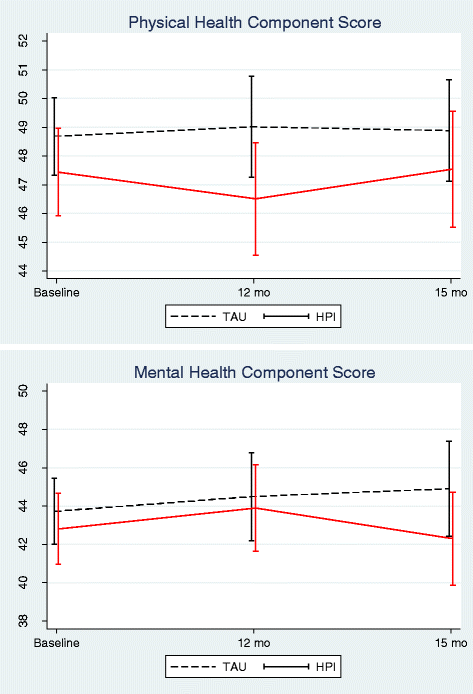
Results: Of 104 care coordinators recruited, 52 (with 213 patients) were randomised to deliver IMPaCT therapy and 52 (with 193 patients) randomised to TAU. Of 406 patients, 318 (78%) and 301 (74%) attended 12- and 15-month follow-up respectively. IMPaCT therapy showed no significant effect on the physical or mental health component SF-36 scores versus TAU at 12 or 15 months. No effect was observed for cardiovascular risk indicators, except for HDL cholesterol, which improved more with IMPACT therapy than TAU (Treatment effect (95% CI); 0.085 (0.007 to 0.16); p = 0.034). The 22% of patients who received >180 min of IMPACT Therapy in addition to usual care achieved a greater reduction in waist circumference than did controls, which was clinically significant.
Conclusion: Training and supervising community care coordinators to use IMPaCT therapy in patients with psychosis is insufficient to significantly improve physical or mental health quality of life. The search for effective, pragmatic interventions deliverable in health care services continues.
Trial registration: The trial was retrospectively registered with ISRCTN registry on 23/4/2010 at ISRCTN58667926 ; recruitment started on 01/03/2010 with first randomization on 09.08.2010 ISRCTN58667926 .
Keywords: Health promotion intervention; Mortality; Psychosis; Quality of life; Schizophrenia.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was obtained from the joint South London and Maudsley and the Institute of Psychiatry NHS Ethics Committee (REC Ref no 09/HO80/41). Once participants were identified, they were approached by trained researchers who explained the trial and gave out an information leaflet regarding the study for consideration. The researcher then met the potential participant to answer any further questions they may have and learn if they wished to consent. Potential participants lacking capacity were ineligible for inclusion in the trial. Participants gave written informed consent, having been informed that their decision whether or not to participate in the study was purely voluntary and, in the case of patient participants, would have no effect on their clinical care. Participants were also told that they were free to withdraw their consent at any time, without their clinical care being affected.
Consent for publication
Not applicable.
Competing interests
FG has received honoraria for advisory work and lectures or CME activity support from Roche, BMS, Lundbeck, Otsaka, Janssen and Sunovion, is a collaborator on a NHS Innovations project co-funded by Janssen and has a family member with professional links to Lilly and GSK, including shares.
KI, speaker fees for Eli Lilly, Janssen, Sanofi.
RM Honoraria for lectures from Lundbeck, Janssen, and Sunovion.
JE is an employee of Lundbeck.
All other authors declare they have no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. - DOI - PMC - PubMed
-
- Speyer H, Nørgaard HCB, Hjorthøj C, Madsen TA, Drivsholm S, Pisinger C, et al. Protocol for CHANGE: a randomized clinical trial assessing lifestyle coaching plus care coordination versus care coordination alone versus treatment as usual to reduce risks of cardiovascular disease in adults with schizophrenia and abdominal obesity. BMC Psychiatry. 2015;15(1):1–11. doi: 10.1186/s12888-015-0465-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical