Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells
- PMID: 29284495
- PMCID: PMC5747183
- DOI: 10.1186/s13014-017-0941-6
Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells
Abstract
Background: Tumor Treating Fields (TTFields) are an anti-neoplastic treatment modality delivered via application of alternating electric fields using insulated transducer arrays placed directly on the skin in the region surrounding the tumor. A Phase 3 clinical trial has demonstrated the effectiveness of continuous TTFields application in patients with glioblastoma during maintenance treatment with Temozolomide. The goal of this study was to evaluate the efficacy of combining TTFields with radiation treatment (RT) in glioma cells. We also examined the effect of TTFields transducer arrays on RT distribution in a phantom model and the impact on rat skin toxicity.
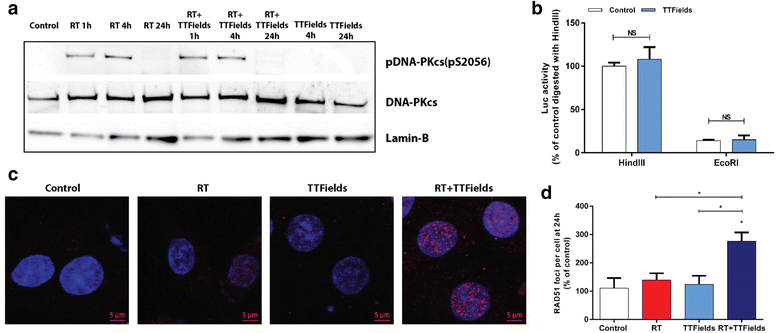
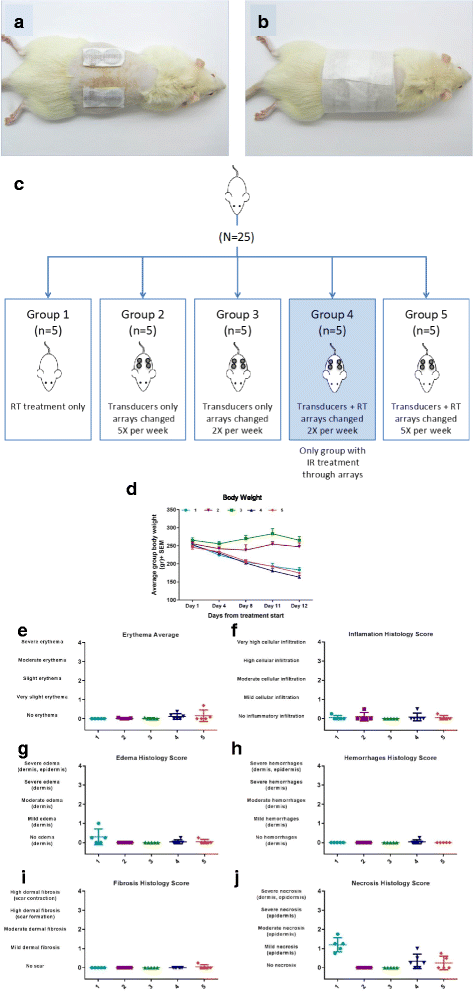
Methods: The efficacy of TTFields application after induction of DNA damage by RT or bleomycin was tested in U-118 MG and LN-18 glioma cells. The alkaline comet assay was used to measure repair of DNA lesions. Repair of DNA double strand breaks (DSBs) were assessed by analyzing γH2AX or Rad51 foci. DNA damage and repair signaled by the activation pattern of phospho-ATM (pS1981) and phospho-DNA-PKcs (pS2056) was evaluated by immunoblotting. The absorption of the RT energy by transducer arrays was measured by applying RT through arrays placed on a solid-state phantom. Skin toxicities were tested in rats irradiated daily through the arrays with 2Gy (total dose of 20Gy).
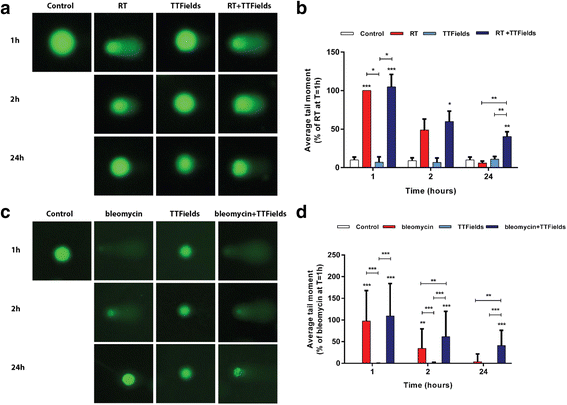
Results: TTFields synergistically enhanced the efficacy of RT in glioma cells. Application of TTFields to irradiated cells impaired repair of irradiation- or chemically-induced DNA damage, possibly by blocking homologous recombination repair. Transducer arrays presence caused a minor reduction in RT intensity at 20 mm and 60 mm below the arrays, but led to a significant increase in RT dosage at the phantom surface jeopardizing the "skin sparing effect". Nevertheless, transducer arrays placed on the rat skin during RT did not lead to additional skin reactions.
Conclusions: Administration of TTFields after RT increases glioma cells treatment efficacy possibly by inhibition of DNA damage repair. These preclinical results support the application of TTFields therapy immediately after RT as a viable regimen to enhance RT outcome. Phantom measurements and animal models imply that it may be possible to leave the transducer arrays in place during RT without increasing skin toxicities.
Keywords: DNA damage repair; Glioma; Radiation treatment; Radiosensitization; TTFields.
Conflict of interest statement
Ethics approval
Ethics approval and consent to participate were obtained from The Israel Board for Animal Experiments.
Consent for publication
Not applicable.
Competing interests
MG, MM, RSS, TV, Yaara Porat, RB, ZB, EDK, UW are paid employees of Novocure.
K Z-C, KV, PH and RL have no conflict of interest associated with this publication.
Yoram Palti holds stock in Novocure Ltd.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321–324. doi: 10.1016/0360-3016(90)90096-3. - DOI - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant TMZ versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

