Accelerated super-resolution imaging with FRET-PAINT
- PMID: 29284498
- PMCID: PMC5747120
- DOI: 10.1186/s13041-017-0344-5
Accelerated super-resolution imaging with FRET-PAINT
Abstract
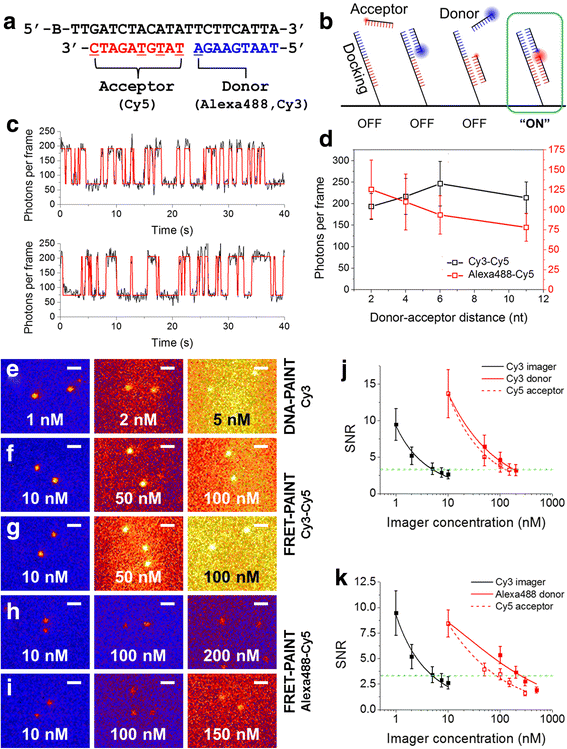
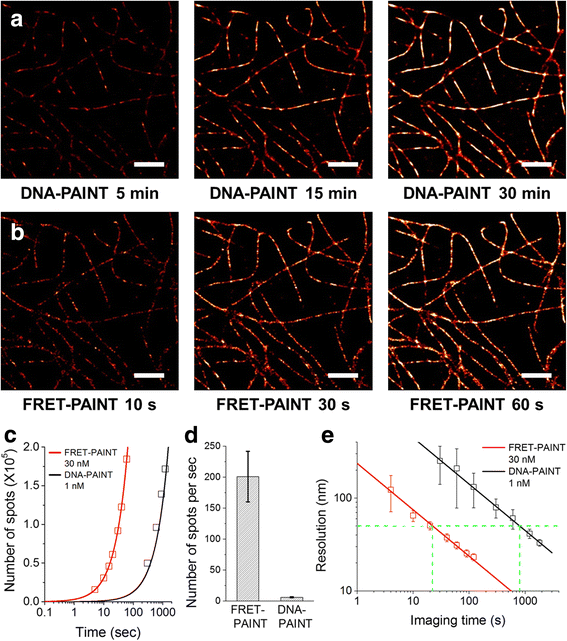
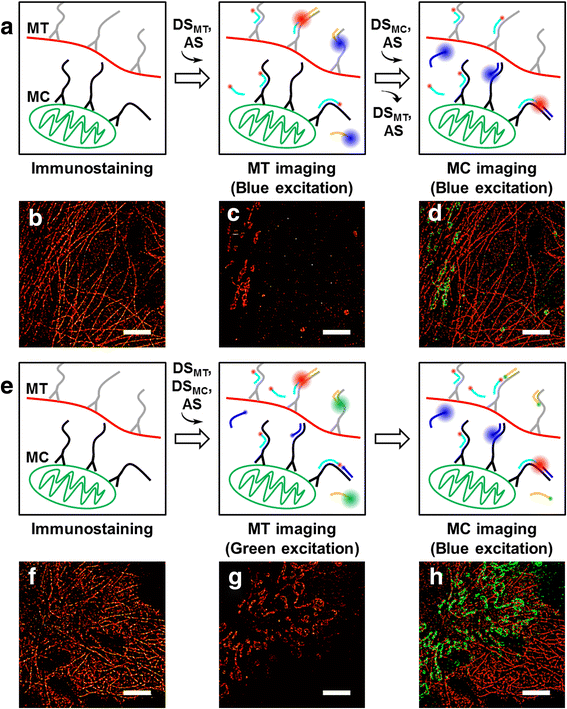
Super-resolution fluorescence microscopy in the current form is hard to be used to image the neural connectivity of thick tissue samples due to problems such as slow imaging speed, severe photobleaching of fluorescent probes, and high background noise. Recently developed DNA-PAINT solved the photobleaching problem, but its imaging speed is extremely low. We report accelerated super-resolution fluorescence microscopy named FRET-PAINT. Compared to conventional DNA-PAINT, the imaging speed of the microscopy increases up to ~30-fold. As demonstrations, we show that 25-50 second imaging time is long enough to provide super-resolution reconstruction of microtubules and mitochondria of COS-7 cells.
Keywords: DNA-PAINT; FRET; FRET-PAINT; SMLM; fluorescence resonance energy transfer; single-molecule localization microscopy; super-resolution microscopy.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hell SW, Kroug M. Ground-state-depletion fluorscence microscopy: A concept for breaking the diffraction resolution limit. Appl. Phys. B. 1995;60:495–497. doi: 10.1007/BF01081333. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

