Bronchoscopy versus an endotracheal tube mounted camera for the peri-interventional visualization of percutaneous dilatational tracheostomy - a prospective, randomized trial (VivaPDT)
- PMID: 29284503
- PMCID: PMC5747130
- DOI: 10.1186/s13054-017-1901-0
Bronchoscopy versus an endotracheal tube mounted camera for the peri-interventional visualization of percutaneous dilatational tracheostomy - a prospective, randomized trial (VivaPDT)
Abstract
Background: Percutaneous dilatational tracheostomy (PDT) in critically ill patients often involves bronchoscopic optical guidance. However, this procedure is not without disadvantages. Therefore, we aimed to study a recently introduced endotracheal tube-mounted camera (VivaSightTM-SL tube [VST]; ETView, Misgav, Israel) for guiding PDT.
Methods: This was a randomized controlled trial involving 46 critically ill patients who received PDT using optical guidance with a VST or with bronchoscopy. The primary outcome measure was visualization of the tracheal structures (i.e., identification and monitoring of the thyroid, cricoid, and tracheal cartilage and the posterior wall) rated on 4-point Likert scales. Secondary measures were the quality of ventilation (before puncture and during the tracheostomy procedure rated on 4-point Likert scales) and blood gases sampled at standardized time points.
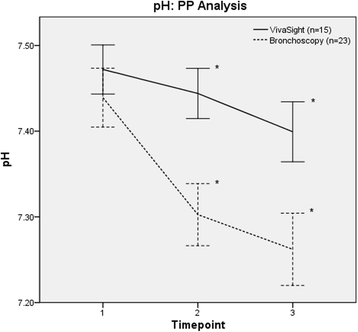
Results: The mean ratings for visualization (lower values better; values given for per-protocol analysis) were 5.4 (95% CI 4.5-6.3) for the VST group and 4.0 (95% CI 4.0-4.0) for the bronchoscopy group (p < 0.001). Mean ventilation ratings were 2.5 (95% CI 2.1-2.9) for VST and 5.0 (95% CI 4.4-5.7) for bronchoscopy (p < 0.001). Arterial carbon dioxide increased to 5.9 (95% CI 5.4-6.5) kPa in the VST group vs. 8.3 (95% CI 7.2-9.5) kPa in the bronchoscopy group (p < 0.001), and pH decreased to 7.40 (95% CI 7.36-7.43) in the VST group vs. 7.26 (95% CI 7.22-7.30) in the bronchoscopy group (p < 0.001), at the end of the intervention.
Conclusions: Visualization of PDT with the VST is not noninferior to guidance by bronchoscopy. Ventilation is superior with less hypercarbia with the VST. Because visualization is not a prerequisite for PDT, patients requiring stable ventilation with normocarbia may benefit from PDT with the VST.
Trial registration: ClinicalTrials.gov, NCT02861001 . Registered on 13 June 2016.
Keywords: Airway management; Artificial; Bronchoscopy; Critical care; Diagnostic equipment; Diagnostic techniques; Hypercapnia; Intratracheal; Intubation; Respiration; Respiratory system; Tracheostomy; Ventilator weaning.
Conflict of interest statement
Ethics approval and consent to participate
The ethics committee of the Hamburg Chamber of Physicians, Germany, approved this study (reference PV5177; March 1, 2016). Written informed consent was obtained from the patients’ legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Feasibility of an endotracheal tube-mounted camera for percutaneous dilatational tracheostomy.Acta Anaesthesiol Scand. 2017 Jul;61(6):660-667. doi: 10.1111/aas.12904. Epub 2017 May 11. Acta Anaesthesiol Scand. 2017. PMID: 28493334
-
Comparison of EtView™ tracheoscopic ventilation tube and video-assisted fiberoptic bronchoscopy during percutaneous dilatational tracheostomy.J Clin Monit Comput. 2017 Jun;31(3):507-512. doi: 10.1007/s10877-016-9885-x. Epub 2016 Apr 29. J Clin Monit Comput. 2017. PMID: 27130402
-
Percutaneous dilatational tracheostomy using a tracheoscopic ventilation tube in an experimental ex vivo animal model.Anaesth Intensive Care. 2016 May;44(3):371-5. doi: 10.1177/0310057X1604400303. Anaesth Intensive Care. 2016. PMID: 27246937
-
Laryngeal mask airway for airway control during percutaneous dilatational tracheostomy.Anaesth Intensive Care. 2011 Nov;39(6):1009-13. doi: 10.1177/0310057X1103900605. Anaesth Intensive Care. 2011. PMID: 22165351 Review.
-
[Tracheotomy - surgical and percutaneous].Zentralbl Chir. 2015 Jun;140(3):339-58; quiz 359-60. doi: 10.1055/s-0035-1546135. Epub 2015 Jul 1. Zentralbl Chir. 2015. PMID: 26131574 Review. German.
Cited by
-
[Airway management in intensive care and emergency medicine : What is new?].Med Klin Intensivmed Notfmed. 2019 May;114(4):334-341. doi: 10.1007/s00063-018-0498-7. Epub 2018 Nov 5. Med Klin Intensivmed Notfmed. 2019. PMID: 30397761 Review. German.
-
Endotracheal tube-mounted camera-assisted intubation versus conventional intubation in intensive care: a prospective, randomised trial (VivaITN).Crit Care. 2018 Sep 22;22(1):235. doi: 10.1186/s13054-018-2152-4. Crit Care. 2018. PMID: 30241488 Free PMC article. Clinical Trial.
-
Modified Technique of Percutaneous Tracheostomy Using Borescope Camera: A Case Series.Indian J Crit Care Med. 2022 Jul;26(7):881-883. doi: 10.5005/jp-journals-10071-24265. Indian J Crit Care Med. 2022. PMID: 36864857 Free PMC article.
-
Evaluation of the Effect of Morphological Structure on Dilatational Tracheostomy Interference Location and Complications with Ultrasonography and Fiberoptic Bronchoscopy.J Clin Med. 2024 May 9;13(10):2788. doi: 10.3390/jcm13102788. J Clin Med. 2024. PMID: 38792330 Free PMC article.
-
Comparison of the percutaneous dilatational tracheostomy with and without flexible bronchoscopy guidance in intensive care units.BMC Anesthesiol. 2025 Mar 31;25(1):142. doi: 10.1186/s12871-025-03022-0. BMC Anesthesiol. 2025. PMID: 40165086 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

