Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL
- PMID: 29284596
- PMCID: PMC5865233
- DOI: 10.1182/blood-2017-09-806521
Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL
Abstract
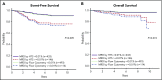
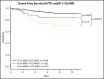
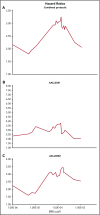
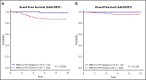
Early response to induction chemotherapy is an important prognostic factor in B-lymphoblastic leukemia (B-ALL). Here, we compare high-throughput sequencing (HTS) of IGH and TRG genes vs flow cytometry (FC) for measurable residual disease (MRD) detection at the end of induction chemotherapy in pediatric patients with newly diagnosed B-ALL. Six hundred nineteen paired pretreatment and end-of-induction bone marrow samples from Children's Oncology Group studies AALL0331 (clinicaltrials.gov #NCT00103285) (standard risk [SR]; with MRD by FC at any level) and AALL0232 (clinicaltrials.gov #NCT00075725) (high risk; with day 29 MRD <0.1% by FC) were evaluated by HTS and FC for event-free (EFS) and overall survival (OS). HTS and FC showed similar 5-year EFS and OS for MRD-positive and -negative patients using an MRD threshold of 0.01%. However, there was a high discordant rate with HTS identifying 55 (38.7%) more patients MRD positive at this threshold. These discrepant patients have worse outcomes than FC MRD-negative patients. In addition, the increased analytic sensitivity of HTS permitted identification of 19.9% of SR patients without MRD at any detectable level who had excellent 5-year EFS (98.1%) and OS (100%). The higher analytic sensitivity and lower false-negative rate of HTS improves upon FC for MRD detection in pediatric B-ALL by identifying a novel subset of patients at end of induction who are essentially cured using current chemotherapy and identifying MRD at 0.01% in up to one-third of patients who are missed at the same threshold by FC.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: H.R. has employment, equity ownership, patents, and royalties with Adaptive Biotechnologies. I.K., B.C., and D. Williamson have employment and equity ownership with Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Figures
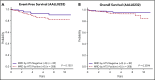
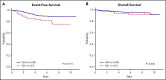
Comment in
-
There is nothing minimal about residual disease.Blood. 2018 Mar 22;131(12):1269-1270. doi: 10.1182/blood-2018-01-824870. Blood. 2018. PMID: 29567755 No abstract available.
References
-
- Pizzo PA, Poplack DG, Adamson PC, Blaney SM, Helman L. Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia: Wolters Kluwer; 2016.
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541-1552. - PubMed
-
- Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127(17):2101-2112. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

