COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin
- PMID: 29284644
- PMCID: PMC5824635
- DOI: 10.1158/1535-7163.MCT-16-0818
COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin
Abstract
Cisplatin-based therapy is highly toxic, but moderately effective in most cancers. Concurrent inhibition of cyclooxygenase-2 (COX-2) and soluble epoxide hydrolase (sEH) results in antitumor activity and has organ-protective effects. The goal of this study was to determine the antitumor activity of PTUPB, an orally bioavailable COX-2/sEH dual inhibitor, in combination with cisplatin and gemcitabine (GC) therapy. NSG mice bearing bladder cancer patient-derived xenografts were treated with vehicle, PTUPB, cisplatin, GC, or combinations thereof. Mouse experiments were performed with two different PDX models. PTUPB potentiated cisplatin and GC therapy, resulting in significantly reduced tumor growth and prolonged survival. PTUPB plus cisplatin was no more toxic than cisplatin single-agent treatment as assessed by body weight, histochemical staining of major organs, blood counts, and chemistry. The combination of PTUPB and cisplatin increased apoptosis and decreased phosphorylation in the MAPK/ERK and PI3K/AKT/mTOR pathways compared with controls. PTUPB treatment did not alter platinum-DNA adduct levels, which is the most critical step in platinum-induced cell death. The in vitro study using the combination index method showed modest synergy between PTUPB and platinum agents only in 5637 cell line among several cell lines examined. However, PTUPB is very active in vivo by inhibiting angiogenesis. In conclusion, PTUPB potentiated the antitumor activity of cisplatin-based treatment without increasing toxicity in vivo and has potential for further development as a combination chemotherapy partner. Mol Cancer Ther; 17(2); 474-83. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures


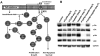
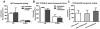
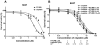
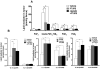
References
-
- Ho GY, Woodward N, Coward JI. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Critical reviews in oncology/hematology. 2016;102:37–46. - PubMed
-
- Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

