Junctional trafficking and restoration of retrograde signaling by the cytoplasmic RyR1 domain
- PMID: 29284662
- PMCID: PMC5806685
- DOI: 10.1085/jgp.201711879
Junctional trafficking and restoration of retrograde signaling by the cytoplasmic RyR1 domain
Abstract
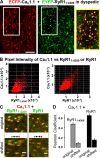
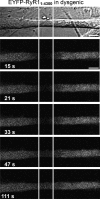
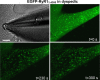
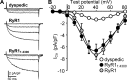
The type 1 ryanodine receptor (RyR1) in skeletal muscle is a homotetrameric protein that releases Ca2+ from the sarcoplasmic reticulum (SR) in response to an "orthograde" signal from the dihydropyridine receptor (DHPR) in the plasma membrane (PM). Additionally, a "retrograde" signal from RyR1 increases the amplitude of the Ca2+ current produced by CaV1.1, the principle subunit of the DHPR. This bidirectional signaling is thought to depend on physical links, of unknown identity, between the DHPR and RyR1. Here, we investigate whether the isolated cytoplasmic domain of RyR1 can interact structurally or functionally with CaV1.1 by producing an N-terminal construct (RyR11:4300) that lacks the C-terminal membrane domain. In CaV1.1-null (dysgenic) myotubes, RyR11:4300 is diffusely distributed, but in RyR1-null (dyspedic) myotubes it localizes in puncta at SR-PM junctions containing endogenous CaV1.1. Fluorescence recovery after photobleaching indicates that diffuse RyR11:4300 is mobile, whereas resistance to being washed out with a large-bore micropipette indicates that the punctate RyR11:4300 stably associates with PM-SR junctions. Strikingly, expression of RyR11:4300 in dyspedic myotubes causes an increased amplitude, and slowed activation, of Ca2+ current through CaV1.1, which is almost identical to the effects of full-length RyR1. Fast protein liquid chromatography indicates that ∼25% of RyR11:4300 in diluted cytosolic lysate of transfected tsA201 cells is present in complexes larger in size than the monomer, and intermolecular fluorescence resonance energy transfer implies that RyR11:4300 is significantly oligomerized within intact tsA201 cells and dyspedic myotubes. A large fraction of these oligomers may be homotetramers because freeze-fracture electron micrographs reveal that the frequency of particles arranged like DHPR tetrads is substantially increased by transfecting RyR-null myotubes with RyR11:4300 In summary, the RyR1 cytoplasmic domain, separated from its SR membrane anchor, retains a tendency toward oligomerization/tetramerization, binds to SR-PM junctions in myotubes only if CaV1.1 is also present and is fully functional in retrograde signaling to CaV1.1.
© 2018 Polster et al.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

