Targeting microRNA/UHRF1 pathways as a novel strategy for cancer therapy
- PMID: 29285183
- PMCID: PMC5738699
- DOI: 10.3892/ol.2017.7290
Targeting microRNA/UHRF1 pathways as a novel strategy for cancer therapy
Abstract
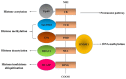
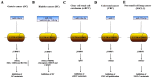
Ubiquitin-like containing plant homeodomain and RING finger domains 1 (UHRF1) is an anti-apoptotic protein involved in the silencing of several tumor suppressor genes (TSGs) through epigenetic modifications including DNA methylation and histone post-translational alterations, and also epigenetic-independent mechanisms. UHRF1 overexpression is observed in a number of solid tumors and hematological malignancies, and is considered a primary mechanism in inhibiting apoptosis. UHRF1 exerts its inhibitory activity on TSGs by binding to functional domains and therefore influences several epigenetic actors including DNA methyltransferase, histone deacetylase 1, histone acetyltransferase Tat-interacting protein 60 and histone methyltransferases G9a and Suv39H1. UHRF1 is considered to control a large macromolecular protein complex termed epigenetic code replication machinery, in order to maintain epigenetic silencing of TSGs during cell division, thus enabling cancer cells to escape apoptosis. MicroRNAs (miRNAs) are able to regulate the expression of its target gene by functioning as either an oncogene or a tumor suppressor. In the present review, the role of tumor suppressive miRNAs in the regulation of UHRF1, and the importance of targeting the microRNA/UHRF1 pathways in order to induce the reactivation of silenced TSGs and subsequent apoptosis are discussed.
Keywords: DNA methylation; apoptosis; cancer; epigenetics; microRNA; tumor suppressor genes; ubiquitin-like with PHD and RING finger domains 1.
Figures
References
-
- Achour M, Jacq X, Rondé P, Alhosin M, Charlot C, Chataigneau T, Jeanblanc M, Macaluso M, Giordano A, Hughes AD, et al. The interaction of the SRA domain of ICBP90 with a novel domain of DNMT1 is involved in the regulation of VEGF gene expression. Oncogene. 2008;27:2187–2197. doi: 10.1038/sj.onc.1210855. - DOI - PubMed
-
- Berkyurek AC, Suetake I, Arita K, Takeshita K, Nakagawa A, Shirakawa M, Tajima S. The DNA methyltransferase Dnmt1 directly interacts with the SET and RING finger-associated (SRA) domain of the multifunctional protein Uhrf1 to facilitate accession of the catalytic center to hemi-methylated DNA. J Biol Chemistry. 2014;289:379–386. doi: 10.1074/jbc.M113.523209. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
