A population pharmacokinetic model for individualised dosage regimens of vancomycin in Chinese neonates and young infants
- PMID: 29285245
- PMCID: PMC5739632
- DOI: 10.18632/oncotarget.22114
A population pharmacokinetic model for individualised dosage regimens of vancomycin in Chinese neonates and young infants
Abstract
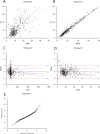
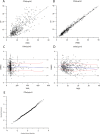
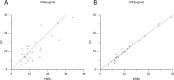
Population pharmacokinetic (PPK) modelling is an easy and impartment method for estimating drug concentration for use inindividualized therapy, especially for young patients and to help protect drug-induced diseases. The purpose of this study was to develop a PPK model for effective dosing of vancomycin in Chinese neonates and young infants. The PPK modelling tool Phoenix® NLME™ was use to assess demographic and routine clinical pharmacokinetic (PK) data retrospectively collected for patients admitted to Children's Hospital of Chongqing Medical University between 2011 and 2016. Data of patients admitted to the hospital between January and June of 2017 were used in validation study, and the final model was also preliminary validated in 2 cases in another hospital. A total of 421 serum samples from 316 patients were included in the initial PPK analysis. A two-compartment PPK model was developed, and exponential-error model was used to describe inter-individual variability of clearance. Residual variability was described by an additive model. The final PPK model was demonstrated as valid by internal and external model evaluation. Of note, the clearance and volume of vancomycin in Chinese neonates and young infants may be greater than in Caucasians. Herein, we describe the establishment of an accurate PPK model of vancomycin for Chinese neonates and young infants, which may be useful as a dosing algorithm for this particular paediatric population.
Keywords: Chinese young infants; phoenix NLME; population pharmacokinetics; vancomycin.
Conflict of interest statement
CONFLICTS OF INTEREST All authors declare that they have no conflicts of interest related to their authorship of this paper or publication of this study.
Figures


References
-
- Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clinical Pharmacokinetics. 2012;51:1–13. - PubMed
-
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. - PubMed
-
- Lomaestro BM. Vancomycin dosing and monitoring 2 years after the guidelines. Expert review of anti-infective therapy. 2011;9:657–667. - PubMed
-
- Hall RG, Giuliano CA, Haase KK, Hazlewood KA, Frei CR, Forcade NA, Brouse SD, Bell T, Bedimo RJ, Alvarez CA. Empiric guideline-recommended weight-based vancomycin dosing and mortality in methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. Bmc Infectious Diseases. 2012;12:1–5. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

