Activation of Nrf2 by MIND4-17 protects osteoblasts from hydrogen peroxide-induced oxidative stress
- PMID: 29285281
- PMCID: PMC5739668
- DOI: 10.18632/oncotarget.22360
Activation of Nrf2 by MIND4-17 protects osteoblasts from hydrogen peroxide-induced oxidative stress
Abstract
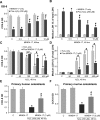
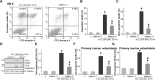
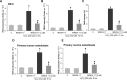
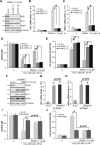
MIND4-17 is a recently developed NF-E2-related factor 2 (Nrf2) activator, which uniquely causes Nrf2 disassociation from Keap1. Here, we showed that pretreatment with MIND4-17 significantly inhibited hydrogen peroxide (H2O2)-induced viability reduction of primary osteoblasts and OB-6 osteoblastic cells. Meanwhile, MIND4-17 inhibited both apoptotic and non-apoptotic osteoblast cell death by H2O2. MIND4-17 treatment induced Keap1-Nrf2 disassociation, causing Nrf2 stabilization, accumulation and nuclear translocation in osteoblasts, leading to transcription of several Nrf2-dependent genes, including heme oxygenase 1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), γ-glutamylcysteine synthetase modifier subunit (GCLM) and catalytic subunit (GCLC). Additionally, MIND4-17 largely attenuated H2O2-reactive oxygen species (ROS) production, lipid peroxidation and DNA damages. Nrf2 knockdown by targeted short hairpin RNA (shRNA) exacerbated H2O2-induced cytotoxicity in OB-6 osteoblastic cells, and nullified MIND4-17-mediated cytoprotection against H2O2. Meanwhile, Keap1 shRNA took over MIND4-17's actions and protected OB-6 cells from H2O2. Together, MIND4-17 activates Nrf2 signaling and protects osteoblasts from H2O2.
Keywords: Keap; MIND4-17; Nrf2; osteoblasts; oxidative stress.
Conflict of interest statement
CONFLICTS OF INTEREST The listed authors have no conflicts of interest.
Figures

Similar articles
-
Four-octyl itaconate activates Nrf2 cascade to protect osteoblasts from hydrogen peroxide-induced oxidative injury.Cell Death Dis. 2020 Sep 17;11(9):772. doi: 10.1038/s41419-020-02987-9. Cell Death Dis. 2020. PMID: 32943614 Free PMC article.
-
MIND4-17 protects retinal pigment epithelium cells and retinal ganglion cells from UV.Oncotarget. 2017 Sep 21;8(52):89793-89801. doi: 10.18632/oncotarget.21131. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163788 Free PMC article.
-
The Nrf2 activator MIND4-17 protects retinal ganglion cells from high glucose-induced oxidative injury.J Cell Physiol. 2020 Oct;235(10):7204-7213. doi: 10.1002/jcp.29619. Epub 2020 Feb 5. J Cell Physiol. 2020. PMID: 32020639
-
Four-octyl itaconate activates Keap1-Nrf2 signaling to protect neuronal cells from hydrogen peroxide.Cell Commun Signal. 2018 Nov 15;16(1):81. doi: 10.1186/s12964-018-0294-2. Cell Commun Signal. 2018. PMID: 30442144 Free PMC article.
-
A novel Keap1 inhibitor iKeap1 activates Nrf2 signaling and ameliorates hydrogen peroxide-induced oxidative injury and apoptosis in osteoblasts.Cell Death Dis. 2021 Jul 5;12(7):679. doi: 10.1038/s41419-021-03962-8. Cell Death Dis. 2021. PMID: 34226516 Free PMC article.
Cited by
-
Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke.Oxid Med Cell Longev. 2018 Nov 6;2018:3172480. doi: 10.1155/2018/3172480. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30533170 Free PMC article.
-
Four-octyl itaconate activates Nrf2 cascade to protect osteoblasts from hydrogen peroxide-induced oxidative injury.Cell Death Dis. 2020 Sep 17;11(9):772. doi: 10.1038/s41419-020-02987-9. Cell Death Dis. 2020. PMID: 32943614 Free PMC article.
-
New strategy of bone marrow mesenchymal stem cells against oxidative stress injury via Nrf2 pathway: oxidative stress preconditioning.J Cell Biochem. 2019 Dec;120(12):19902-19914. doi: 10.1002/jcb.29298. Epub 2019 Jul 26. J Cell Biochem. 2019. PMID: 31347718 Free PMC article.
-
Nrf2 signaling activation by a small molecule activator compound 16 inhibits hydrogen peroxide-induced oxidative injury and death in osteoblasts.Cell Death Discov. 2022 Aug 8;8(1):353. doi: 10.1038/s41420-022-01146-7. Cell Death Discov. 2022. PMID: 35941127 Free PMC article.
-
Maqui Berry and Ginseng Extracts Reduce Cigarette Smoke-Induced Cell Injury in a 3D Bone Co-Culture Model.Antioxidants (Basel). 2022 Dec 14;11(12):2460. doi: 10.3390/antiox11122460. Antioxidants (Basel). 2022. PMID: 36552669 Free PMC article.
References
-
- Souttou B, Raulais D, Vigny M. Pleiotrophin induces angiogenesis: involvement of the phosphoinositide-3 kinase but not the nitric oxide synthase pathways. J Cell Physiol. 2001;187:59–64. - PubMed
-
- Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, Lee KW, Kang MI. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010;87:226–235. - PubMed
-
- Tare RS, Oreffo RO, Sato K, Rauvala H, Clarke NM, Roach HI. Effects of targeted overexpression of pleiotrophin on postnatal bone development. Biochem Biophys Res Commun. 2002;298:324–332. - PubMed
-
- Fan JB, Liu W, Zhu XH, Yuan K, Xu DW, Chen JJ, Cui ZM. EGFR-AKT-mTOR activation mediates epiregulin-induced pleiotropic functions in cultured osteoblasts. Mol Cell Biochem. 2015;398:105–113. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

