Ginseng Rh2 protects endometrial cells from oxygen glucose deprivation/re-oxygenation
- PMID: 29285285
- PMCID: PMC5739672
- DOI: 10.18632/oncotarget.22390
Ginseng Rh2 protects endometrial cells from oxygen glucose deprivation/re-oxygenation
Abstract
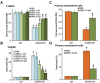
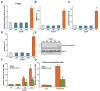
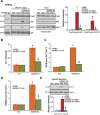
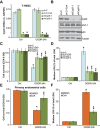
In this study, oxygen glucose deprivation/re-oxygenation (OGDR) was applied to cultured endometrial cells to mimic ischemic-reperfusion injuries. We also tested the potential effect of Ginseng Rh2 (GRh2) against the process. In established T-HESC human endometrial cells and primary murine endometrial cells, GRh2 largely inhibited OGDR-induced viability reduction and cell death. Remarkably, OGDR induced programmed necrosis in the endometrial cells, evidenced by cyclophilin D-p53-adenine nucleotide translocator 1 (ANT-1) mitochondrial association, mitochondrial depolarization, reactive oxygen species production, and lactate dehydrogenase release. Notably, such effects by OGDR were largely attenuated with co-treatment of GRh2. Further, cyclophilin D inhibition or knockdown also protected endometrial cells from OGDR. On the other hand, forced over-expression of cyclophilin D facilitated OGDR-induced T-HESC cell necrosis, which was dramatically inhibited by GRh2. Together, GRh2 protects endometrial cells from OGDR possibly via inhibiting CypD-dependent programmed necrosis pathway.
Keywords: Ginseng Rh2; cyclophilin; endometrial cells; oxygen glucose deprivation/re-oxygenation; programmed necrosis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no competing interest.
Figures
Similar articles
-
microRNA-1203 targets and silences cyclophilin D to protect human endometrial cells from oxygen and glucose deprivation-re-oxygenation.Aging (Albany NY). 2020 Feb 10;12(3):3010-3024. doi: 10.18632/aging.102795. Epub 2020 Feb 10. Aging (Albany NY). 2020. PMID: 32041924 Free PMC article.
-
Targeting cyclophilin-D by compound 19 protects neuronal cells from oxygen glucose deprivation/re-oxygenation.Oncotarget. 2017 Oct 6;8(52):90238-90249. doi: 10.18632/oncotarget.21655. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163824 Free PMC article.
-
Ciliary Neurotrophic Factor (CNTF) Protects Myocardial Cells from Oxygen Glucose Deprivation (OGD)/Re-Oxygenation via Activation of Akt-Nrf2 Signaling.Cell Physiol Biochem. 2018;51(4):1852-1862. doi: 10.1159/000495711. Epub 2018 Nov 30. Cell Physiol Biochem. 2018. PMID: 30504707
-
Keratinocyte growth factor protects endometrial cells from oxygen glucose deprivation/re-oxygenation via activating Nrf2 signaling.Biochem Biophys Res Commun. 2018 Jun 18;501(1):178-185. doi: 10.1016/j.bbrc.2018.04.208. Epub 2018 May 5. Biochem Biophys Res Commun. 2018. PMID: 29709474
-
The ways for ginsenoside Rh2 to fight against cancer: the molecular evidences in vitro and in vivo.J Ginseng Res. 2023 Mar;47(2):173-182. doi: 10.1016/j.jgr.2022.09.011. Epub 2022 Oct 6. J Ginseng Res. 2023. PMID: 36926617 Free PMC article. Review.
Cited by
-
The histone acetyltransferase HBO1 functions as a novel oncogenic gene in osteosarcoma.Theranostics. 2021 Mar 4;11(10):4599-4615. doi: 10.7150/thno.55655. eCollection 2021. Theranostics. 2021. PMID: 33754016 Free PMC article.
-
Keap1-Nrf2 signaling activation by Bardoxolone-methyl ameliorates high glucose-induced oxidative injury in human umbilical vein endothelial cells.Aging (Albany NY). 2020 Jun 2;12(11):10370-10380. doi: 10.18632/aging.103263. Epub 2020 Jun 2. Aging (Albany NY). 2020. PMID: 32484788 Free PMC article.
-
CPI-1189 protects neuronal cells from oxygen glucose deprivation/re-oxygenation-induced oxidative injury and cell death.Aging (Albany NY). 2021 Feb 17;13(5):6712-6723. doi: 10.18632/aging.202528. Epub 2021 Feb 17. Aging (Albany NY). 2021. PMID: 33621193 Free PMC article.
-
AMPK activation by ASP4132 inhibits non-small cell lung cancer cell growth.Cell Death Dis. 2021 Apr 6;12(4):365. doi: 10.1038/s41419-021-03655-2. Cell Death Dis. 2021. PMID: 33824293 Free PMC article.
-
Neuron-secreted NLGN3 ameliorates ischemic brain injury via activating Gαi1/3-Akt signaling.Cell Death Dis. 2023 Oct 25;14(10):700. doi: 10.1038/s41419-023-06219-8. Cell Death Dis. 2023. PMID: 37880221 Free PMC article.
References
-
- Mullins TL, Miller RJ, Mullins ES. Evaluation and management of adolescents with abnormal uterine bleeding. Pediatr Ann. 2015;44:e218–22. https://doi.org/10.3928/00904481-20150910-09 - DOI - PubMed
-
- Van de Velde M, Diez C, Varon AJ. Obstetric hemorrhage. Curr Opin Anaesthesiol. 2015;28:186–90. https://doi.org/10.1097/ACO.0000000000000168 - DOI - PubMed
-
- Deering S, Rowland J. Obstetric emergency simulation. Semin Perinatol. 2013;37:179–88. https://doi.org/10.1053/j.semperi.2013.02.010 - DOI - PubMed
-
- Ferrari RS, Andrade CF. Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev. 2015;2015:590987. https://doi.org/10.1155/2015/590987 - DOI - PMC - PubMed
-
- Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–14. https://doi.org/10.1016/j.redox.2014.05.006 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

