Exploring the effect of primary tumor sidedness on therapeutic efficacy across treatment lines in patients with metastatic colorectal cancer: analysis of FIRE-3 (AIOKRK0306)
- PMID: 29285289
- PMCID: PMC5739676
- DOI: 10.18632/oncotarget.22396
Exploring the effect of primary tumor sidedness on therapeutic efficacy across treatment lines in patients with metastatic colorectal cancer: analysis of FIRE-3 (AIOKRK0306)
Abstract
Purpose: To assess the impact of primary tumor sidedness on outcome of patients with metastatic colorectal cancer (mCRC) across treatment lines.
Patients and methods: Patients of the FIRE-3 trial (initial FOLFIRI plus either cetuximab or bevacizumab) were separately evaluated according to primary tumor site differentiating left-sided (LPT) from right-sided primary tumors (RPT). Efficacy (i.e. progression-free survival (PFS2nd) and overall survival (OS2nd) of second-line therapy) was evaluated by Kaplan-Meier method and compared by log rank test as well as Cox regression analyses. All analyses were also reported according to drug sequences.
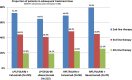
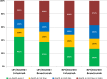
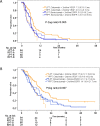
Results: 411 of 592 patients (69%) with KRAS exon 2 wild-type tumors received 2nd-line therapy has and had available information on primary tumor location, of those 309 patients (75%) presented with LPT. In patients with LPT, PFS2nd was markedly longer than in patients with RPT (6.0 months [95% CI 5.5-6.5] versus 3.8 months [95% CI 2.5-5.2], hazard ratio: 0.61 [95% CI 0.47-0.78], P<0.001). Differences in PFS2nd between study-arms were evident in patients with LPT, but not in patients with RPT (Cox model interaction test, P=0.12). Consistent observations were also made for OS2nd.
Conclusion: This retrospective analysis of FIRE-3 indicates that efficacy of second-line therapy was significantly greater in patients with left-sided tumors as compared to right-sided tumors. This difference was driven by superior activity of second-line regimens of the initial cetuximab-arm as compared to the initial bevacizumab-arm in left-sided tumors. Our observations confirm the strong prognostic value of primary tumor location in second-line therapy of mCRC.
Keywords: EGFR antibody; bevacizumab; colorectal cancer; sequential therapy; tumor sidedness.
Conflict of interest statement
CONFLICTS OF INTEREST DPM: Honoraria: Merck, Amgen, Bayer, Servier, Roche, MSD, SIRTEX. Travel Support: Merck, Roche, Amgen, Bayer, Sanofi, Servier. SS: Honoraria (Talks & Advisory boards): Amgen, Merck, Roche, Lilly, Bayer, Sanofi. LFvW: none. TD: none. AK: Honoraria: Merck, Roche. UVK: Honoraria: Gilead, MSD, Abbvie, Roche Pharma, Lilly, Amgen. SEAB: Advisory boards: Merck, Roche. Research grant: Roche. TH: none. CK:none. GS:none. FK: Honoraria/advisory boards: BMS, Celgene, Merck, Lilly, Merck, Sanofi, TevaWS. MM: Honoraria/travel support: Lilly, Amgen, Roche, Merck, MSD, BMS, Pfizer, AstraZeneca. JWH: honoraria for advisory board and speaker from Roche and travel support from Novartis. JCvE: Travel support: Apceth. SH: none. VH: Honoraria: Merck, Amgen, Roche, Sanofi, SIRTEX. Consulting or Advisory Board: Merck, Amgen, Roche, Sanofi, SIRTEX. Research funding: Merck, Amgen, Roche, SanofiTravel Accommodation expenses: Merck, Roche.
Figures

References
-
- Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. - PubMed
-
- Modest DP, Schulz C, von Weikersthal LF, Quietzsch D, von Einem JC, Schalhorn A, Vehling-Kaiser U, Laubender RP, Giessen C, Stintzing S, Heinemann V. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment) Anticancer Drugs. 2014;25:212–218. - PubMed
-
- von Einem JC, Heinemann V, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, Decker T, Klein S, Held S, Jung A, Kirchner T, Haas M, Holch J, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140:1607–1614. - PMC - PubMed
-
- Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–1729. - PMC - PubMed
-
- Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ, Heinemann V. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2016 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

