HLA-G+3027 polymorphism is associated with tumor relapse in pediatric Hodgkin's lymphoma
- PMID: 29285306
- PMCID: PMC5739693
- DOI: 10.18632/oncotarget.22515
HLA-G+3027 polymorphism is associated with tumor relapse in pediatric Hodgkin's lymphoma
Abstract
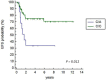
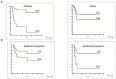
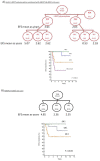
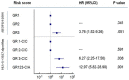
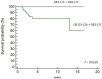
In this study, we tested whether polymorphisms in human leukocyte antigen G (HLA-G) were associated with event-free survival (EFS) in pediatric Hodgkin's lymphoma (HL). We evaluated the association of HLA-G 3'-UTR polymorphisms with EFS in 113 pediatric HL patients treated using the AIEOP LH-2004 protocol. Patients with the +3027-C/A genotype (rs17179101, UTR-7 haplotype) showed lower EFS than those with the +3027-C/C genotype (HR= 3.23, 95%CI: 0.99-10.54, P=0.012). Female patients and systemic B symptomatic patients with the HLA-G +3027 polymorphism showed lower EFS. Multivariate analysis showed that the +3027-A polymorphism (HR 3.17, 95%CI 1.16-8.66, P=0.025) was an independent prognostic factor. Immunohistochemical analysis showed that HL cells from patients with the +3027-C/A genotype did not express HLA-G. Moreover, HLA-G +3027 polymorphism improved EFS prediction when added to the algorithm for therapeutic group classification of pediatric HL patients. Our findings suggest HLA-G +3027 polymorphism is a prognostic marker in pediatric HL patients undergoing treatment according to LH-2004 protocol.
Keywords: +3027 C/A genotype; 3’UTR polymorphism; HLA-G; event-free survival; pediatric hodgkin lymphoma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that there are no conflicts of interest.
Figures

References
-
- AIRTUM Working Group. CCM. AIEOP Working Group [Article in English, Italian]. Italian cancer figures, report 2012: cancer in children and adolescents. Epidemiol Prev. 2013;37:1–225. - PubMed
-
- Janz M, Mathas S. The pathogenesis of classical Hodgkin's lymphoma: what can we learn from analyses of genomic alterations in Hodgkin and Reed-Sternberg cells? Haematologica. 2008;93:1292–5. https://doi.org/10.3324/haematol.13614 - DOI - PubMed
-
- Boiocchi M, De Re V, Dolcetti R, Carbone A, Scarpa A, Menestrina F. Association of Epstein-Barr virus genome with mixed cellularity and cellular phase nodular sclerosis Hodgkin's disease subtypes. Ann Oncol. 1992;3:307–10. - PubMed
-
- Farruggia P, Puccio G, Sala A, Todesco A, Buffardi S, Garaventa A, Bottigliero G, Bianchi M, Zecca M, Locatelli F, Pession A, Pillon M, Favre C, et al. The prognostic value of biological markers in paediatric Hodgkin lymphoma. Eur J Cancer. 2016;52:33–40. https://doi.org/10.1016/j.ejca.2015.09.003 - DOI - PubMed
-
- Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, Stovall M, Robison LL, Hudson MM. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–16. https://doi.org/10.1182/blood-2010-04-278796 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

