Characterization and correction of intraoperative soft tissue deformation in image-guided laparoscopic liver surgery
- PMID: 29285519
- PMCID: PMC5729961
- DOI: 10.1117/1.JMI.5.2.021203
Characterization and correction of intraoperative soft tissue deformation in image-guided laparoscopic liver surgery
Abstract
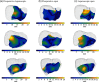
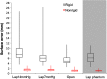
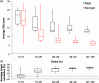
Laparoscopic liver surgery is challenging to perform due to a compromised ability of the surgeon to localize subsurface anatomy in the constrained environment. While image guidance has the potential to address this barrier, intraoperative factors, such as insufflation and variable degrees of organ mobilization from supporting ligaments, may generate substantial deformation. The severity of laparoscopic deformation in humans has not been characterized, and current laparoscopic correction methods do not account for the mechanics of how intraoperative deformation is applied to the liver. We first measure the degree of laparoscopic deformation at two insufflation pressures over the course of laparoscopic-to-open conversion in 25 patients. With this clinical data alongside a mock laparoscopic phantom setup, we report a biomechanical correction approach that leverages anatomically load-bearing support surfaces from ligament attachments to iteratively reconstruct and account for intraoperative deformations. Laparoscopic deformations were significantly larger than deformations associated with open surgery, and our correction approach yielded subsurface target error of [Formula: see text] and surface error of [Formula: see text] using only sparse surface data with realistic surgical extent. Laparoscopic surface data extents were examined and found to impact registration accuracy. Finally, we demonstrate viability of the correction method with clinical data.
Keywords: biomechanical modeling; image-guided surgery; laparoscopy; liver; registration; soft tissue deformation.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

