Molecular regulation of epithelial-to-mesenchymal transition in tumorigenesis (Review)
- PMID: 29286071
- PMCID: PMC5819928
- DOI: 10.3892/ijmm.2017.3320
Molecular regulation of epithelial-to-mesenchymal transition in tumorigenesis (Review)
Abstract
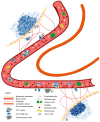
Numerous studies over the past two decades have focused on the epithelial‑to‑mesenchymal transition (EMT) and its role in the development of metastasis. Certain studies highlighted the importance of EMT in the dissemination of tumor cells and metastasis of epithelium‑derived carcinomas. Tumor metastasis is a multistep process during which tumor cells change their morphology, and start to migrate and invade distant sites. The present review discusses the current understanding of the molecular mechanisms contributing to EMT in embryogenesis, fibrosis and tumorigenesis. Additionally, the signaling pathways that initiate EMT through transcriptional factors responsible for the activation and suppression of various genes associated with cancer cell migration were investigated. Furthermore, the important role of the epigenetic modifications that regulate EMT and the reverse process, mesenchymal‑to‑epithelial transition (MET) are discussed. MicroRNAs are key regulators of various intracellular processes and current knowledge of EMT has significantly improved due to microRNA characterization. Their effect on signaling pathways and the ensuing events that occur during EMT at the molecular level is becoming increasingly recognized. The current review also highlights the role of circulating tumor cells (CTCs) and CTC clusters, and their ability to form metastases. In addition, the biological properties of different types of circulating cells based on their tumor‑forming potential are compared.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

