Cigarette smoke-induced EGFR activation promotes epithelial mesenchymal migration of human retinal pigment epithelial cells through regulation of the FAK-mediated Syk/Src pathway
- PMID: 29286114
- PMCID: PMC5802154
- DOI: 10.3892/mmr.2017.8355
Cigarette smoke-induced EGFR activation promotes epithelial mesenchymal migration of human retinal pigment epithelial cells through regulation of the FAK-mediated Syk/Src pathway
Abstract
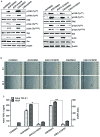
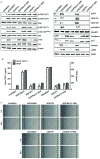
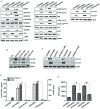
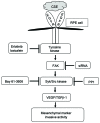
Epithelial-mesenchymal transition (EMT) of retinal pigment epithelial (RPE) cells is inevitable change of age‑related macular degeneration (AMD). Smoking is a major risk factor for the development of EMT in several diseases, including lung cancer. Cigarette smoke‑induced stress promotes the production of epidermal growth factor (EGF) in RPE cells. However, the underlying signaling pathways induced by aberrant EGF receptor (EGFR) expression in cigarette smoke-exposed RPE cells remain largely unknown. In the present study, the morphological transformation and production of EMT-associated cytokines were investigated to analyze the effect of smoking on the retina. Furthermore, EGF‑treated or cigarette smoke‑exposed RPE cells, as well as the downstream targets of EGFR, were investigated to identify the key molecules involved in EMT of cigarette smoke‑stimulated RPE cells via immunoblotting. Exposure of RPE cells to cigarette smoke extract (CSE) induced secretion of VEGF and TGF‑β1, and increased the expression of EMT markers. CSE‑mediated focal adhesion kinase (FAK) activation resulted in the phosphorylation and activation of spleen associated tyrosine kinase (Syk)/Src proto‑oncogene, non‑receptor tyrosine kinase (Src), leading to migration and invasion of RPE cells. Knockdown of FAK or pharmacological inhibition of Syk/Src abrogated CSE‑mediated VEGF and TGF‑β1 production and blocked the phosphorylation of Smad2/3 in CSE‑stimulated RPE cells. Erlotinib (an EGFR inhibitor) suppressed EGF and CSE‑mediated switch from an epithelial to mesenchymal phenotype. Baicalein, an inhi-bitor of 12/15‑lipooxygenase, also efficiently suppressed CSE‑induced EMT processes by inhibiting EGFR‑associated downstream signaling transduction. The results identified a novel signaling pathway mediated by EGFR in CSE‑activated RPE cells, and suggest baicalein as a potential new therapeutic drug for CSE‑associated retinopathy.
Keywords: retinal pigment epithelial cell; cigarette smoke; focal adhesion kinase; epidermal growth factor receptor; epithelial mesenchymal transition; 12/15-lipooxygenase.
Figures




Similar articles
-
Inhibition of oxidative stress-induced epithelial-mesenchymal transition in retinal pigment epithelial cells of age-related macular degeneration model by suppressing ERK activation.J Adv Res. 2024 Jun;60:141-157. doi: 10.1016/j.jare.2023.06.004. Epub 2023 Jun 15. J Adv Res. 2024. PMID: 37328058 Free PMC article.
-
MMP-2 and MMP-9 mediate cigarette smoke extract-induced epithelial-mesenchymal transition in airway epithelial cells via EGFR/Akt/GSK3β/β-catenin pathway: Amelioration by fisetin.Chem Biol Interact. 2019 Dec 1;314:108846. doi: 10.1016/j.cbi.2019.108846. Epub 2019 Oct 10. Chem Biol Interact. 2019. PMID: 31606474
-
A novel aminothiazole KY-05009 with potential to inhibit Traf2- and Nck-interacting kinase (TNIK) attenuates TGF-β1-mediated epithelial-to-mesenchymal transition in human lung adenocarcinoma A549 cells.PLoS One. 2014 Oct 22;9(10):e110180. doi: 10.1371/journal.pone.0110180. eCollection 2014. PLoS One. 2014. PMID: 25337707 Free PMC article.
-
[Aberrant Activation Mechanism of TGF-β Signaling in Epithelial-mesenchymal Transition].Yakugaku Zasshi. 2021;141(11):1229-1234. doi: 10.1248/yakushi.21-00143. Yakugaku Zasshi. 2021. PMID: 34719542 Review. Japanese.
-
Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review.
Cited by
-
Network Pharmacology-Based Identification of Key Targets of Ziyin Mingmu Pills Acting on Age-Related Macular Degeneration.Evid Based Complement Alternat Med. 2023 Feb 2;2023:5933125. doi: 10.1155/2023/5933125. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 36777624 Free PMC article.
-
Age- and sex- divergent translatomic responses of the mouse retinal pigmented epithelium.Neurobiol Aging. 2024 Aug;140:41-59. doi: 10.1016/j.neurobiolaging.2024.04.012. Epub 2024 May 3. Neurobiol Aging. 2024. PMID: 38723422 Free PMC article.
-
Water-pipe smoking promotes epithelial-mesenchymal transition and invasion of human breast cancer cells via ERK1/ERK2 pathways.Cancer Cell Int. 2018 Nov 13;18:180. doi: 10.1186/s12935-018-0678-9. eCollection 2018. Cancer Cell Int. 2018. PMID: 30473629 Free PMC article.
-
Inhibitory effect of nintedanib on VEGF secretion in retinal pigment epithelial cells induced by exposure to a necrotic cell lysate.PLoS One. 2019 Aug 6;14(8):e0218632. doi: 10.1371/journal.pone.0218632. eCollection 2019. PLoS One. 2019. PMID: 31386668 Free PMC article.
-
Myofibroblasts in macular fibrosis secondary to neovascular age-related macular degeneration - the potential sources and molecular cues for their recruitment and activation.EBioMedicine. 2018 Dec;38:283-291. doi: 10.1016/j.ebiom.2018.11.029. Epub 2018 Nov 22. EBioMedicine. 2018. PMID: 30473378 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

