Mutual regulation of the Hippo/Wnt/LPA/TGF‑β signaling pathways and their roles in glaucoma (Review)
- PMID: 29286147
- PMCID: PMC5819904
- DOI: 10.3892/ijmm.2017.3352
Mutual regulation of the Hippo/Wnt/LPA/TGF‑β signaling pathways and their roles in glaucoma (Review)
Abstract
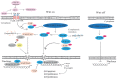
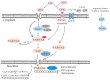
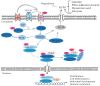
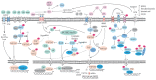
Glaucoma is the leading cause of irreversible blindness worldwide and there is no effective treatment thus far. The trabecular meshwork has been identified as the major pathological area involved. Certain signaling pathways in the trabecular meshwork, including the Wnt, lysophosphatidic acid and transforming growth factor‑β pathways, have been identified as novel therapeutic targets in glaucoma treatment. Meanwhile, it has been reported that key proteins in these pathways, particularly the primary transcription regulator Yes‑associated protein (YAP) and transcriptional co‑activator with PDZ‑binding motif (TAZ), exhibit interactions with the Hippo pathway. The Hippo pathway, which was first identified in Drosophila, has drawn great focus with regard to various aspects of studies in recent years. One role of the Hippo pathway in the regulation of organ size was indicated by more recent evidence. Defining the relevant physiological function of the Hippo pathway has proven to be extremely complicated. Studies have ascribed a role for the Hippo pathway in an overwhelming number of processes, including cell proliferation, cell death and cell differentiation. Therefore, the present review aimed to unravel the roles of YAP and TAZ in the Hippo pathway and the pathogenesis of glaucoma. Furthermore, a new and creative study for the treatment of glaucoma is provided.
Figures

References
-
- Knepper PA, Goossens W, Hvizd M, Palmberg PF. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1996;37:1360–1367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

