Molecular structure of human KATP in complex with ATP and ADP
- PMID: 29286281
- PMCID: PMC5790381
- DOI: 10.7554/eLife.32481
Molecular structure of human KATP in complex with ATP and ADP
Abstract
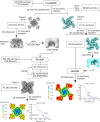
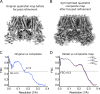
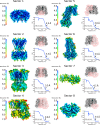
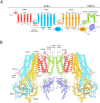
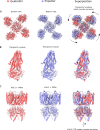
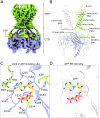
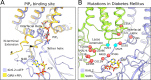
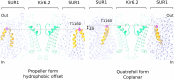
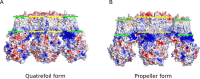
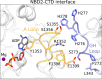
In many excitable cells, KATP channels respond to intracellular adenosine nucleotides: ATP inhibits while ADP activates. We present two structures of the human pancreatic KATP channel, containing the ABC transporter SUR1 and the inward-rectifier K+ channel Kir6.2, in the presence of Mg2+ and nucleotides. These structures, referred to as quatrefoil and propeller forms, were determined by single-particle cryo-EM at 3.9 Å and 5.6 Å, respectively. In both forms, ATP occupies the inhibitory site in Kir6.2. The nucleotide-binding domains of SUR1 are dimerized with Mg2+-ATP in the degenerate site and Mg2+-ADP in the consensus site. A lasso extension forms an interface between SUR1 and Kir6.2 adjacent to the ATP site in the propeller form and is disrupted in the quatrefoil form. These structures support the role of SUR1 as an ADP sensor and highlight the lasso extension as a key regulatory element in ADP's ability to override ATP inhibition.
Keywords: ADP; ATP; KATP; Kir6.2; SUR1; biophysics; cryo-EM; neuroscience; none; structural biology.
© 2017, Lee et al.
Conflict of interest statement
KL, JC, RM No competing interests declared
Figures








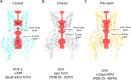



References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Aittoniemi J, Fotinou C, Craig TJ, de Wet H, Proks P, Ashcroft FM. Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:257–267. doi: 10.1098/rstb.2008.0142. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

