Simulations for designing and interpreting intervention trials in infectious diseases
- PMID: 29287587
- PMCID: PMC5747936
- DOI: 10.1186/s12916-017-0985-3
Simulations for designing and interpreting intervention trials in infectious diseases
Abstract
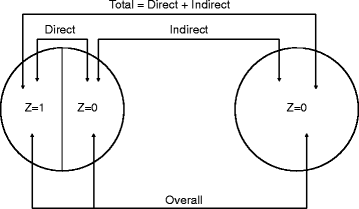
Background: Interventions in infectious diseases can have both direct effects on individuals who receive the intervention as well as indirect effects in the population. In addition, intervention combinations can have complex interactions at the population level, which are often difficult to adequately assess with standard study designs and analytical methods.
Discussion: Herein, we urge the adoption of a new paradigm for the design and interpretation of intervention trials in infectious diseases, particularly with regard to emerging infectious diseases, one that more accurately reflects the dynamics of the transmission process. In an increasingly complex world, simulations can explicitly represent transmission dynamics, which are critical for proper trial design and interpretation. Certain ethical aspects of a trial can also be quantified using simulations. Further, after a trial has been conducted, simulations can be used to explore the possible explanations for the observed effects.
Conclusion: Much is to be gained through a multidisciplinary approach that builds collaborations among experts in infectious disease dynamics, epidemiology, statistical science, economics, simulation methods, and the conduct of clinical trials.
Keywords: Clinical trial design; Infectious diseases; Mathematical modeling; Simulations; Vaccine.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Ross R. An application of the theory of probabilities to the study of a priori pathometry. Part 1. Proc R Soc Series A. 1916;92:204–30. doi: 10.1098/rspa.1916.0007. - DOI
-
- Baird S, Bohren J, McIntosh C, Özler B. Optimal design of experiments in the presence of interference. Review of Economics and Statistics. Accepted August 7, 2017. doi:10.1162/REST_a_00716, in Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

