Tobacco dependence treatment in the emergency department: A randomized trial using the Multiphase Optimization Strategy
- PMID: 29287665
- PMCID: PMC5851600
- DOI: 10.1016/j.cct.2017.12.016
Tobacco dependence treatment in the emergency department: A randomized trial using the Multiphase Optimization Strategy
Abstract
Background: Tobacco dependence remains the leading preventable cause of death in the developed world. Smokers are disproportionately from lower socioeconomic groups, and may use the hospital emergency department (ED) as an important source of care. A recent clinical trial demonstrated the efficacy of a multicomponent intervention to help smokers quit, but the independent contributions of those components is unknown.
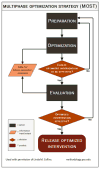
Methods: This is a full-factorial (16-arm) randomized trial in a busy hospital ED of 4 tobacco dependence interventions: brief motivational interviewing, nicotine replacement therapy, referral to a telephone quitline, and a texting program. The trial utilizes the Multiphase Optimization Strategy (MOST) and a novel mixed methods analytic design to assess clinical efficacy, cost effectiveness, and qualitative participant feedback. The primary endpoint is tobacco abstinence at 3months, verified by participants' exhaled carbon monoxide.
Results: Study enrollment began in February 2017. As of April 2017, 52 of 1056 planned participants (4.9%) were enrolled. Telephone-based semi-structured participant interviews and in-person biochemical verification of smoking abstinence are completed at the 3-month follow-up. Efficacy and cost effectiveness analyses will be conducted after follow-up is completed.
Discussion: The goal of this study is to identify a clinically efficacious, cost-effective intervention package for the initial treatment of tobacco dependence in ED patients. The efficacy of this combination can then be tested in a subsequent confirmatory trial. Our approach incorporates qualitative feedback from study participants in evaluating which intervention components will be tested in the future trial.
Trial registration: Trial (NCT02896400) registered in ClinicalTrials.gov on September 6, 2016.
Keywords: Emergency department; Mixed methods; Smoking cessation; Tobacco dependence treatment.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Toll received a grant from Pfizer for medicine only, and he testifies as an expert witness for plaintiffs who have filed litigation against the tobacco companies.
All other authors declare that they have no competing interests.
Figures
References
-
- U.S. Department of Health and Human Services. A report of the surgeon general. U.S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. The health consequences of smoking— 50 years of progress.
-
- Office of Disease Prevention and Health Promotion. Tobacco Use: Objectives. U.S. Department of Health and Human Services; [Accessed 15 December 2014]. 2010. https://www.healthypeople.gov/2020/topicsobjectives/topic/tobacco-use/ob....
-
- NIH State-of-the-Science Panel. National Institutes of Health state-of-the-science conference statement: tobacco use: prevention, cessation, and control. Ann Intern Med. 2006;145:839–844. - PubMed
-
- Centers for Disease Control and Prevention, National Hospital Ambulatory Medical Care Survey. [Accessed 15 December 2014];Emergency Department Summary Tables. 2011 at http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous