Angiogenesis and lymphangiogenesis in corneal transplantation-A review
- PMID: 29287709
- PMCID: PMC6003844
- DOI: 10.1016/j.survophthal.2017.12.008
Angiogenesis and lymphangiogenesis in corneal transplantation-A review
Abstract
Corneal transplantation has been proven effective for returning the gift of sight to those affected by corneal disorders such as opacity, injury, and infections that are a leading cause of blindness. Immune privilege plays an important role in the success of corneal transplantation procedures; however, immune rejection reactions do occur, and they, in conjunction with a shortage of corneal donor tissue, continue to pose major challenges. Corneal immune privilege is important to the success of corneal transplantation and closely related to the avascular nature of the cornea. Corneal avascularity may be disrupted by the processes of angiogenesis and lymphangiogenesis, and for this reason, these phenomena have been a focus of research in recent years. Through this research, therapies addressing certain rejection reactions related to angiogenesis have been developed and implemented. Corneal donor tissue shortages also have been addressed by the development of new materials to replace the human donor cornea. These advancements, along with other improvements in the corneal transplantation procedure, have contributed to an improved success rate for corneal transplantation. We summarize recent developments and improvements in corneal transplantation, including the current understanding of angiogenesis mechanisms, the anti-angiogenic and anti-lymphangiogenic factors identified to date, and the new materials being used. Additionally, we discuss future directions for research in corneal transplantation.
Keywords: VEGF; VEGFR; angiogenesis; biomaterial; corneal neovascularization; corneal transplantation; lymphangiogenesis; stem cell.
Published by Elsevier Inc.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures















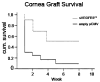
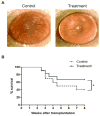

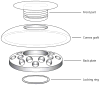
References
-
- Abdel-Naby W, Liu A, Wey N, Rosenblatt MI. Silk fibroin substrates support corneal epithelial cellular adhesion under shear stress. Invest Ophthalmol Vis Sci. 2014;55:4633–4633.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

