Long-term early development research in congenital heart disease (LEADER-CHD): a study protocol for a prospective cohort observational study investigating the development of children after surgical correction for congenital heart defects during the first 3 years of life
- PMID: 29288186
- PMCID: PMC5770821
- DOI: 10.1136/bmjopen-2017-018966
Long-term early development research in congenital heart disease (LEADER-CHD): a study protocol for a prospective cohort observational study investigating the development of children after surgical correction for congenital heart defects during the first 3 years of life
Abstract
Introduction: Congenital heart disease (CHD) is the most common birth defect. Studies on the development of children with CHD point towards deficits in motoric, cognitive and language development. However, most studies are cross-sectional and there is a gap in the knowledge concerning developmental trajectories, risk and protective factors and a lack of research concerning environmental predictors. Specifically, no studies have so far considered the importance of early caregiving experiences and child temperament for the development of children with CHD.
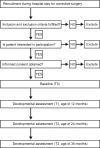
Methods: In a single-centre prospective cohort study, cognitive, motoric and language development of 180 children after corrective surgery for a simple transposition of the great arteries (TGA), tetralogy of Fallot (TOF) or ventricular septal defect (VSD) will be assessed at ages 12, 24 and 36 months with the Bayley Scales of Infant Development 3rd Edition (BSID-III). At age 12 months, a free-play video observation will be conducted to investigate the relationship between primary caregiver and child, and child temperament will be assessed with the Infant Behavior Questionnaire-Revised Short Version. Medical information will be obtained from patient records and demographic information via questionnaires.
Analysis: Frequency and severity of developmental delays will be reported descriptively. Differences between groups (TGA, TOF, VSD) will be subjected to repeated-measures analysis across time points. Multiple regressions will be applied for the analysis of predictors at each time point. For the analysis of differential developmental trajectories, mixed-model analysis will be applied.
Ethics and dissemination: The study has been approved by the local medical ethics committee. Written informed consent will be obtained from all participants. Parents have the option to be debriefed about BSID-III results after each assessment and about the study results after project completion. Results will be disseminated in peer-reviewed journals and presented at conferences.
Trial registration number: DRKS00011006; Pre-results.
Keywords: congenital heart disease; paediatric cardiac surgery; paediatric cardiology.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control: World Health Organization, 2011.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous