Can we S ave the rectum by watchful waiting or T rans A nal microsurgery following (chemo) R adiotherapy versus T otal mesorectal excision for early RE ctal C ancer (STAR-TREC study)?: protocol for a multicentre, randomised feasibility study
- PMID: 29288190
- PMCID: PMC5770914
- DOI: 10.1136/bmjopen-2017-019474
Can we S ave the rectum by watchful waiting or T rans A nal microsurgery following (chemo) R adiotherapy versus T otal mesorectal excision for early RE ctal C ancer (STAR-TREC study)?: protocol for a multicentre, randomised feasibility study
Abstract
Introduction: Total mesorectal excision (TME) is the highly effective standard treatment for rectal cancer but is associated with significant morbidity and may be overtreatment for low-risk cancers. This study is designed to determine the feasibility of international recruitment in a study comparing organ-saving approaches versus standard TME surgery.
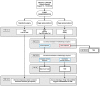
Methods and analysis: STAR-TREC trial is a multicentre international randomised, three-arm parallel, phase II feasibility study in patients with biopsy-proven adenocarcinoma of the rectum. The trial is coordinated from Birmingham, UK with national hubs in Radboudumc (the Netherlands) and Odense University Hospital Svendborg UMC (Denmark). Patients with rectal cancer, staged by CT and MRI as ≤cT3b (up to 5 mm of extramural spread) N0 M0 can be included. Patients will be randomised to either standard TME surgery (control), organ-saving treatment using long-course concurrent chemoradiation or organ-saving treatment using short-course radiotherapy. For patients treated with an organ-saving strategy, clinical response to (chemo)radiotherapy determines the next treatment step. An active surveillance regime will be performed in the case of a complete clinical regression. In the case of incomplete clinical regression, patients will proceed to local excision using an optimised platform such as transanal endoscopic microsurgery or other transanal techniques (eg, transanal endoscopic operation or transanal minimally invasive surgery). The primary endpoint of this phase II study is to demonstrate sufficient international recruitment in order to sustain a phase III study incorporating pelvic failure as the primary endpoint. Success in phase II is defined as randomisation of at least four cases per month internationally in year 1, rising to at least six cases per month internationally during year 2.
Ethics and dissemination: The medical ethical committees of all the participating countries have approved the study protocol. Results of the primary and secondary endpoints will be submitted for publication in peer-reviewed journals.
Trial registration number: ISRCTN14240288, 20 October 2016. NCT02945566; Pre-results, October 2016.
Keywords: TEM; chemoradiation; radiotherapy; rectal cancer; watchful waiting.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
