Extracellular Lactate: A Novel Measure of T Cell Proliferation
- PMID: 29288205
- PMCID: PMC5776880
- DOI: 10.4049/jimmunol.1700886
Extracellular Lactate: A Novel Measure of T Cell Proliferation
Abstract
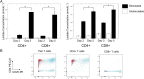
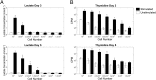
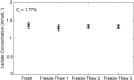
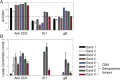
Following activation, T cells rapidly divide and acquire effector functions. This energetically demanding process depends upon the ability of T cells to undergo metabolic remodeling from oxidative phosphorylation to aerobic glycolysis, during which glucose is converted into lactate and released extracellularly. In this article, we demonstrate that extracellular lactate can be used to dynamically assess human T cell responses in vitro. Extracellular lactate levels strongly correlated with T cell proliferation, and measuring lactate compared favorably with traditional methods for determining T cell responses (i.e., [3H]thymidine incorporation and the use of cell proliferation dyes). Furthermore, we demonstrate the usefulness of measuring lactate as a read-out in conventional suppression assays and high-throughput peptide-screening assays. Extracellular lactate was stably produced over 7 d, and results were reproducibly performed over several freeze-thaw cycles. We conclude that the use of extracellular lactate measurements can be a sensitive, safe, stable, and easy-to-implement research tool for measuring T cell responses and cellular metabolic changes in vitro.
Copyright © 2018 The Authors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

