Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial
- PMID: 29288237
- PMCID: PMC5968824
- DOI: 10.1158/1078-0432.CCR-17-2994
Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial
Abstract
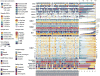
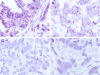
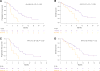
Purpose: To perform real-time whole genome sequencing (WGS) and RNA sequencing (RNASeq) of advanced pancreatic ductal adenocarcinoma (PDAC) to identify predictive mutational and transcriptional features for better treatment selection.Experimental Design: Patients with advanced PDAC were prospectively recruited prior to first-line combination chemotherapy. Fresh tumor tissue was acquired by image-guided percutaneous core biopsy for WGS and RNASeq. Laser capture microdissection was performed for all cases. Primary endpoint was feasibility to report WGS results prior to first disease assessment CT scan at 8 weeks. The main secondary endpoint was discovery of patient subsets with predictive mutational and transcriptional signatures.Results: Sixty-three patients underwent a tumor biopsy between December 2015 and June 2017. WGS and RNASeq were successful in 62 (98%) and 60 (95%), respectively. Genomic results were reported at a median of 35 days (range, 19-52 days) from biopsy, meeting the primary feasibility endpoint. Objective responses to first-line chemotherapy were significantly better in patients with the classical PDAC RNA subtype compared with those with the basal-like subtype (P = 0.004). The best progression-free survival was observed in those with classical subtype treated with m-FOLFIRINOX. GATA6 expression in tumor measured by RNA in situ hybridization was found to be a robust surrogate biomarker for differentiating classical and basal-like PDAC subtypes. Potentially actionable genetic alterations were found in 30% of patients.Conclusions: Prospective genomic profiling of advanced PDAC is feasible, and our early data indicate that chemotherapy response differs among patients with different genomic/transcriptomic subtypes. Clin Cancer Res; 24(6); 1344-54. ©2017 AACR.
Trial registration: ClinicalTrials.gov NCT02750657.
©2017 American Association for Cancer Research.
Conflict of interest statement
S. Moura reports receiving speakers bureau honoraria from Celgene Corporation. J.M.S. Bartlett holds ownership interest (including patents) in 61 Due North, BioNTech, Biotheranostics, Insight Genetics, and Oncology Education, and is a consultant/advisory board member for BioNTech, Biotheranostics, and Insight Genetics. J.J. Yeh and R.A. Moffitt have a pending patent #15/518,900 with United States Patent and Trademark Office on methods and compositions for prognostic and/or diagnostic subtyping of pancreatic cancer. No potential conflicts of interest were disclosed by the other authors.
Figures


References
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. - PubMed
-
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. - PubMed
-
- Dreyer SB, Chang DK, Bailey P, Biankin AV. Pancreatic cancer genomes: implications for clinical management and therapeutic development. Clin Cancer Res. 2017;23:1638–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

