PI3K/Akt Pathway: A Role in δ-Opioid Receptor-Mediated RGC Neuroprotection
- PMID: 29288267
- PMCID: PMC5749243
- DOI: 10.1167/iovs.16-20673
PI3K/Akt Pathway: A Role in δ-Opioid Receptor-Mediated RGC Neuroprotection
Abstract
Purpose: This study examines the role of PI3K/Akt pathway in δ-opioid receptor agonist (SNC-121)-induced RGC neuroprotection in a chronic glaucoma rat model.
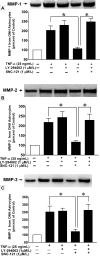
Methods: Injecting hypertonic saline into the limbal veins of Brown Norway rats elevated IOP. Rats were treated either with 1 mg/kg SNC-121 or 3 mg/kg 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride (LY-294002; PI3K/Akt inhibitor) plus SNC-121 once daily for 7 days. Pattern ERGs were recorded in response to contrast reversal of patterned visual stimuli. Retinal ganglion cells (RGC) were visualized by Fluorogold retrograde labeling. Optic nerve head (ONH) astrocytes were pretreated with PI3K/Akt inhibitors for 30 minutes followed by 1-μM SNC-121 treatment. Changes in matrix metalloproteinases (MMP-1, -2, and -3) production and PI3K/Akt activation in optic nerve and TNF-α treated ONH astrocytes were measured by immunohistochemistry and Western blotting.
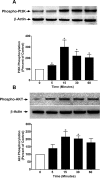
Results: SNC-121 activates the PI3K/Akt pathway in ONH astrocytes and the retina. In ONH astrocytes, SNC-121-induced Akt activation was fully inhibited by PI3K/Akt inhibitors. A sustained decline (7-42 days post injury) in Akt activation was seen in the ocular-hypertensive retina and optic nerve. This decline is reversed to normal levels by 1-mg/kg intraperitoneally (i.p.) SNC-121 treatment. Both pattern ERG amplitudes and RGC numbers were reduced in ocular hypertensive eyes, which were significantly increased in SNC-121-treated animals. Interestingly, SNC-121-induced increase in pattern-ERG amplitudes and RGC numbers were inhibited in LY-294002 pretreated animals. Additionally, SNC-121 treatment inhibited MMP-1, -2, and -3 production from the optic nerve of ocular hypertensive rats and TNF-α-treated ONH astrocytes.
Conclusions: PI3K/Akt pathway plays a crucial role in SNC-121-mediated RGC neuroprotection against glaucomatous injury.
Figures








References
-
- Boland MV, Quigley HA. . Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007; 16: 406– 418. - PubMed
-
- Tezel G, Li LY, Patil RV, Wax MB. . TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001; 42: 1787– 1794. - PubMed
-
- Zhou X, Li F, Kong L, Tomita H, Li C, Cao W. . Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J Biol Chem. 2005; 280: 31240– 31248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

