Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens
- PMID: 29288513
- PMCID: PMC5834780
- DOI: 10.1111/cas.13498
Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens
Abstract
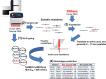
Utilizing the host immune system to eradicate cancer cells has been the most investigated subject in the cancer research field in recent years. However, most of the studies have focused on highly variable responses from immunotherapies such as immune checkpoint inhibitors, from which the majority of patients experienced no or minimum clinical benefit. Advances in genomic sequencing technologies have improved our understanding of immunopharmacogenomics and allowed us to identify novel cancer-specific immune targets. Highly tumor-specific antigens, neoantigens, are generated by somatic mutations that are not present in normal cells. It is plausible that by targeting antigens with high tumor-specificity, such as neoantigens, the likelihood of toxic effects is very limited. However, understanding the interaction between neoantigens and the host immune system remains a significant challenge. This review focuses on the potential use of neoantigen-targeted immunotherapies in cancer treatment and the recent progress of different strategies in predicting, identifying, and validating neoantigens. Successful identification of highly tumor-specific antigens accelerates the development of personalized immunotherapy with no or minimum adverse effects and with a broader coverage of patients.
Keywords: T-cell receptor repertoire; cancer precision medicine; immune checkpoint inhibitor; neoantigen; next-generation sequencing.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

References
-
- Galon J, Costes A, Sanchez‐Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960‐1964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

