Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin
- PMID: 29289646
- PMCID: PMC5985226
- DOI: 10.1016/j.molmet.2017.12.006
Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin
Erratum in
-
Corrigendum to "Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin" [Molecular Metabolism 8 (2018) 1-12].Mol Metab. 2018 Aug;14:158. doi: 10.1016/j.molmet.2018.05.018. Epub 2018 Jun 4. Mol Metab. 2018. PMID: 29886180 Free PMC article. No abstract available.
Abstract
Objectives: The autonomic nervous system is critically involved in mediating the control by leptin of many physiological processes. Here, we examined the role of the leptin receptor (LepR) in proopiomelanocortin (POMC) and agouti-related peptide (AgRP) neurons in mediating the effects of leptin on regional sympathetic and parasympathetic nerve activity.
Methods: We analyzed how deletion of the LepR in POMC neurons (POMCCre/LepRfl/fl mice) or AgRP neurons (AgRPCre/LepRfl/fl mice) affects the ability of leptin to increase sympathetic and parasympathetic nerve activity. We also studied mice lacking the catalytic p110α or p110β subunits of phosphatidylinositol-3 kinase (PI3K) in POMC neurons.
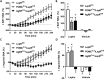
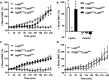
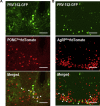
Results: Leptin-evoked increase in sympathetic nerve activity subserving thermogenic brown adipose tissue was partially blunted in mice lacking the LepR in either POMC or AgRP neurons. On the other hand, loss of the LepR in AgRP, but not POMC, neurons interfered with leptin-induced sympathetic nerve activation to the inguinal fat depot. The increase in hepatic sympathetic traffic induced by leptin was also reduced in mice lacking the LepR in AgRP, but not POMC, neurons whereas LepR deletion in either AgRP or POMC neurons attenuated the hepatic parasympathetic nerve activation evoked by leptin. Interestingly, the renal, lumbar and splanchnic sympathetic nerve activation caused by leptin were significantly blunted in POMCCre/LepRfl/fl mice, but not in AgRPCre/LepRfl/fl mice. However, loss of the LepR in POMC or AgRP neurons did not interfere with the ability of leptin to increase sympathetic traffic to the adrenal gland. Furthermore, ablation of the p110α, but not the p110β, isoform of PI3K from POMC neurons eliminated the leptin-elicited renal sympathetic nerve activation. Finally, we show trans-synaptic retrograde tracing of both POMC and AgRP neurons from the kidneys.
Conclusions: POMC and AgRP neurons are differentially involved in mediating the effects of leptin on autonomic nerve activity subserving various tissues and organs.
Keywords: Autonomic nervous system; Cardiovascular regulation; Energy homeostasis; Leptin.
Copyright © 2017 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures




Comment in
-
Central leptin and autonomic regulation: A melanocortin business.Mol Metab. 2018 Feb;8:211-213. doi: 10.1016/j.molmet.2018.01.001. Epub 2018 Jan 9. Mol Metab. 2018. PMID: 29429634 Free PMC article. No abstract available.
References
-
- Friedman J.M., Mantzoros C.S. 20 years of leptin: from the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism. 2015;64:1–4. - PubMed
-
- Plum L., Rother E., Munzberg H., Wunderlich F.T., Morgan D.A., Hampel B. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metabolism. 2007;6:431–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

