New advances in CNS immunity against viral infection
- PMID: 29289900
- PMCID: PMC5990251
- DOI: 10.1016/j.coviro.2017.12.003
New advances in CNS immunity against viral infection
Abstract
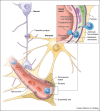
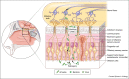
The central nervous system (CNS) is an immunologically specialized organ where restrictive barrier structures protect the parenchyma from inflammation and infection. This protection is important in preventing damage to non-renewable resident cell populations, such as neurons, responsible for functions ranging from executive to autonomic. Despite these barriers, the CNS can be infected through several entry portals, giving rise to meningitis and encephalitis. Following infection, resident cells recruit peripherally derived immune cells to sites of viral infection. In this review, we discuss recent advances in immune recruitment and entry at barrier structures as well as current immunotherapeutic strategies for the treatment of persistent viral infections.
Published by Elsevier B.V.
Figures



References
-
- Riddell J.T., Shuman E.K. Epidemiology of central nervous system infection. Neuroimaging Clin N Am. 2012;22:543–556. - PubMed
-
- Tunkel A.R., Glaser C.A., Bloch K.C., Sejvar J.J., Marra C.M., Roos K.L., Hartman B.J., Kaplan S.L., Scheld W.M., Whitley R.J. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

